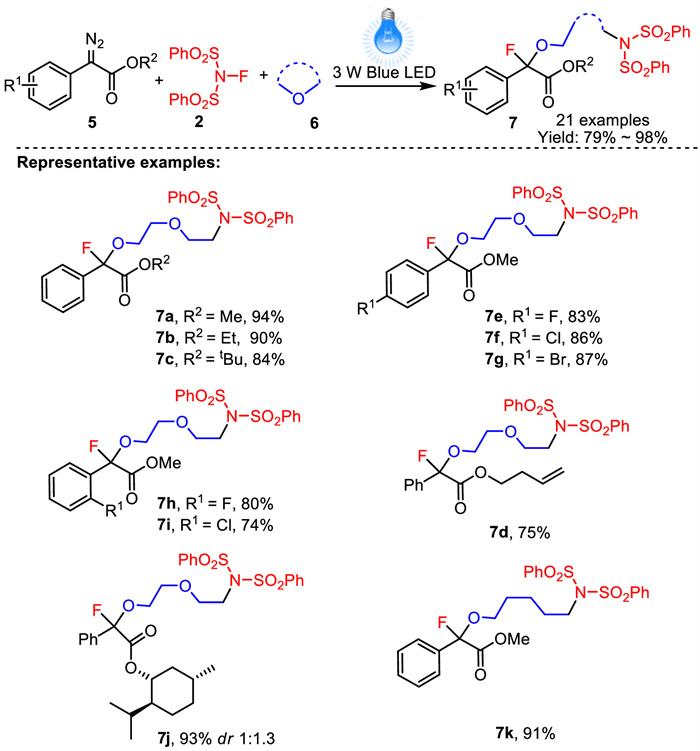
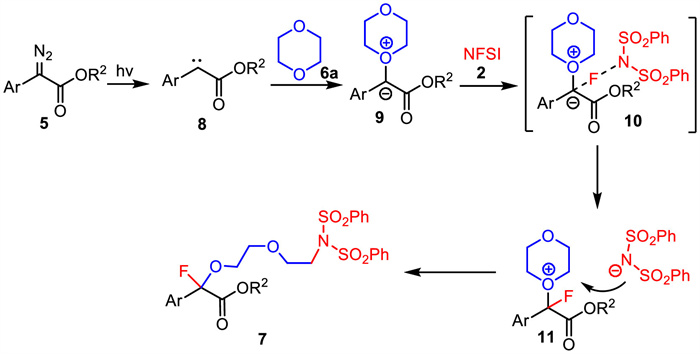
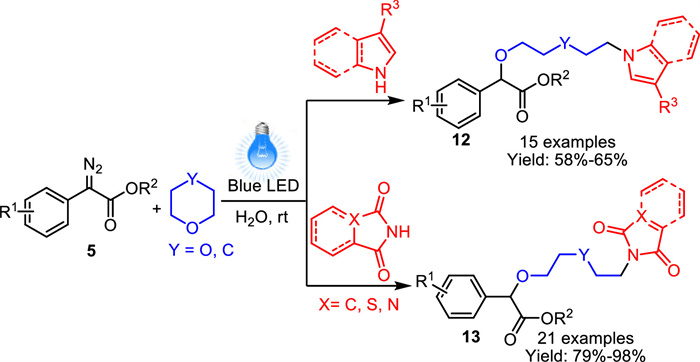
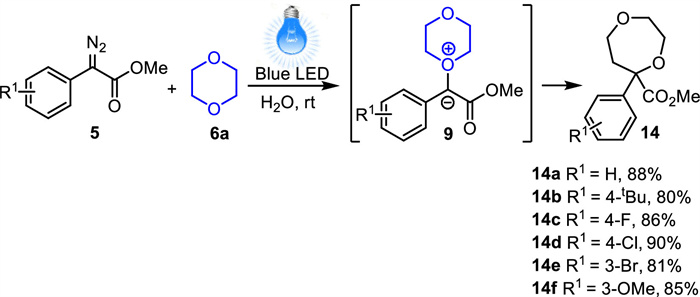
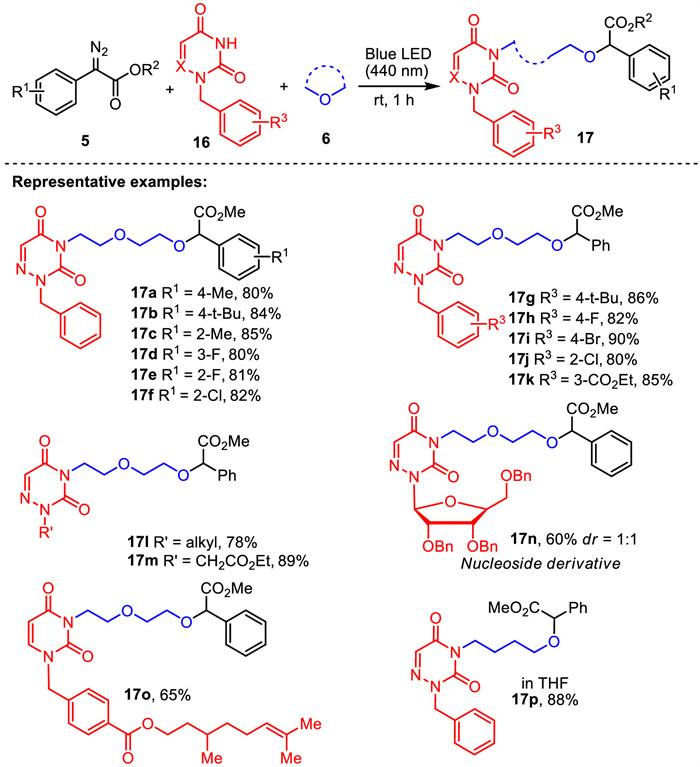
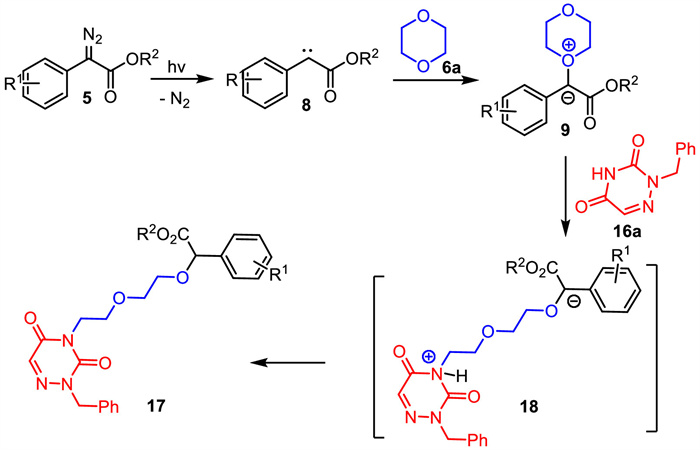
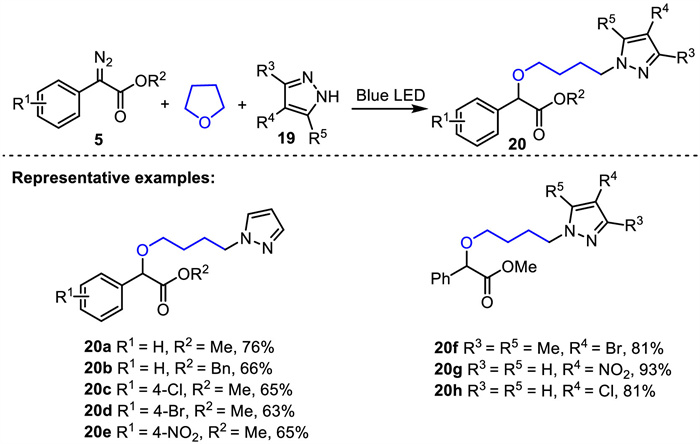
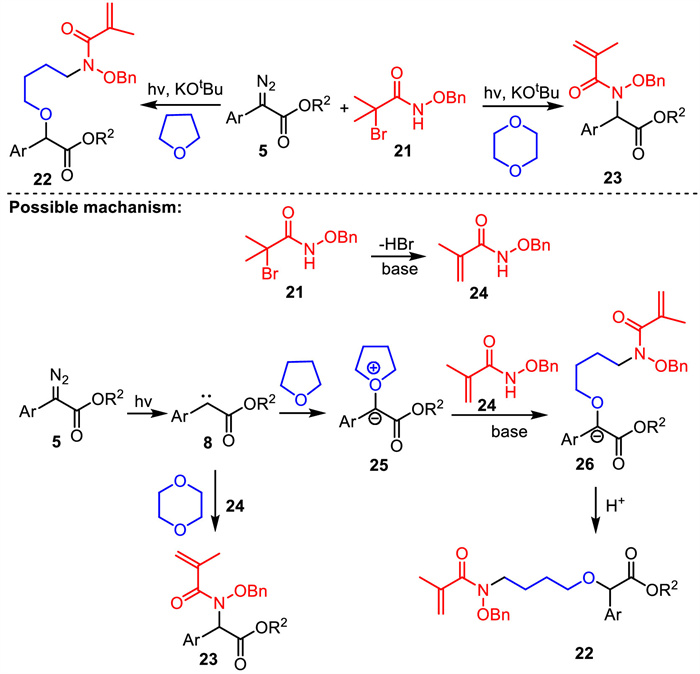
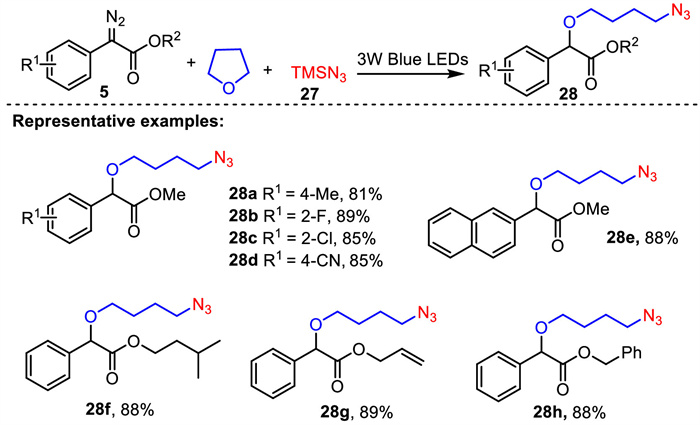
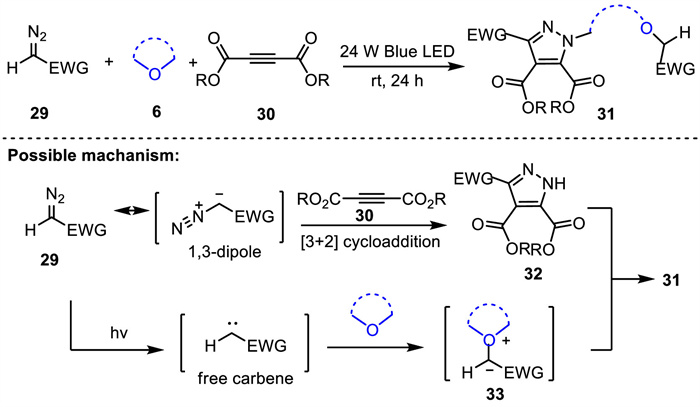
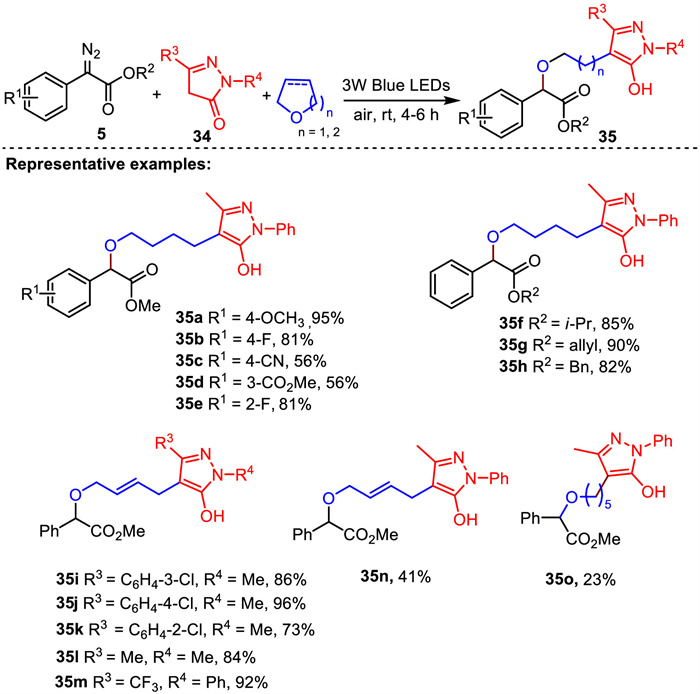
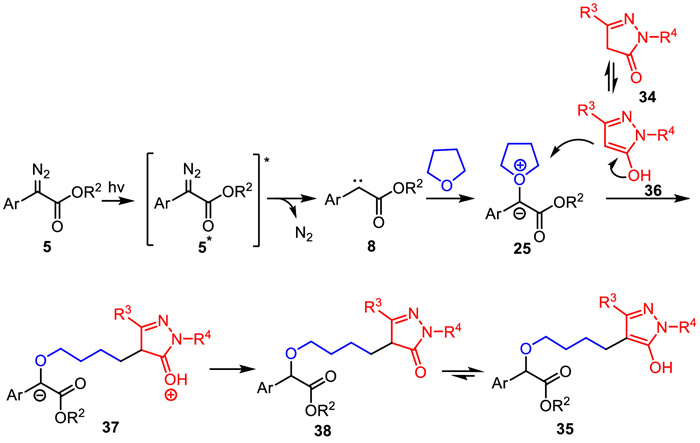
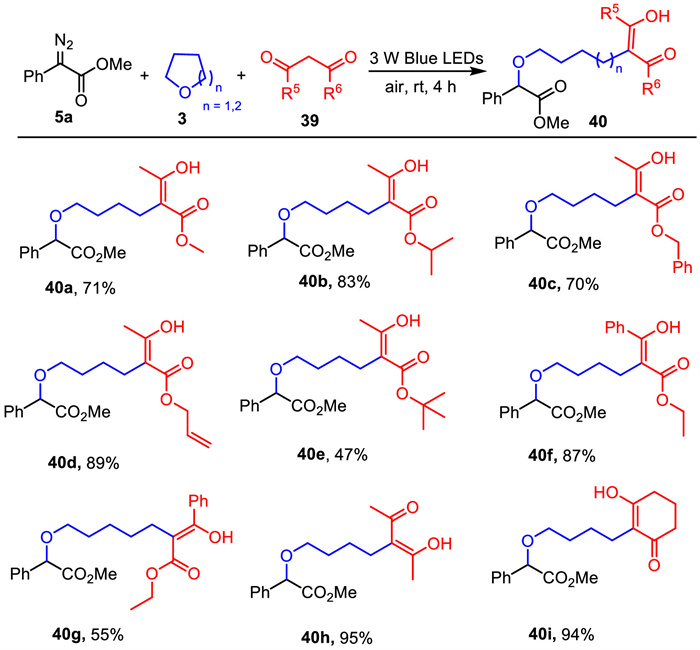
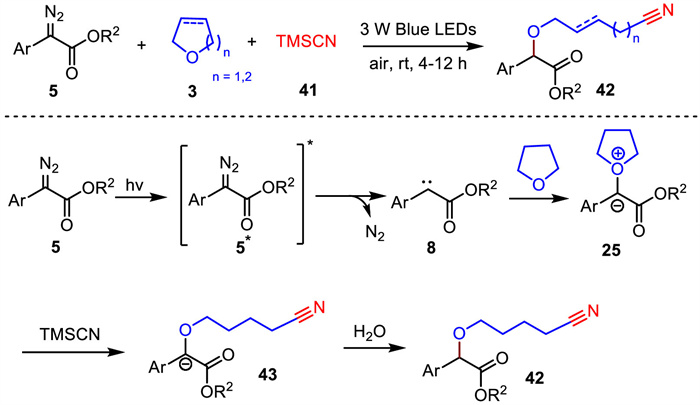
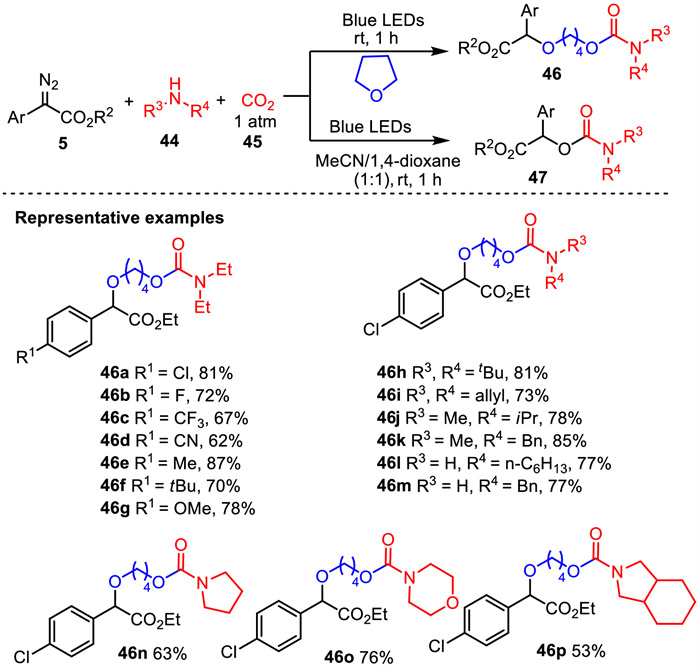
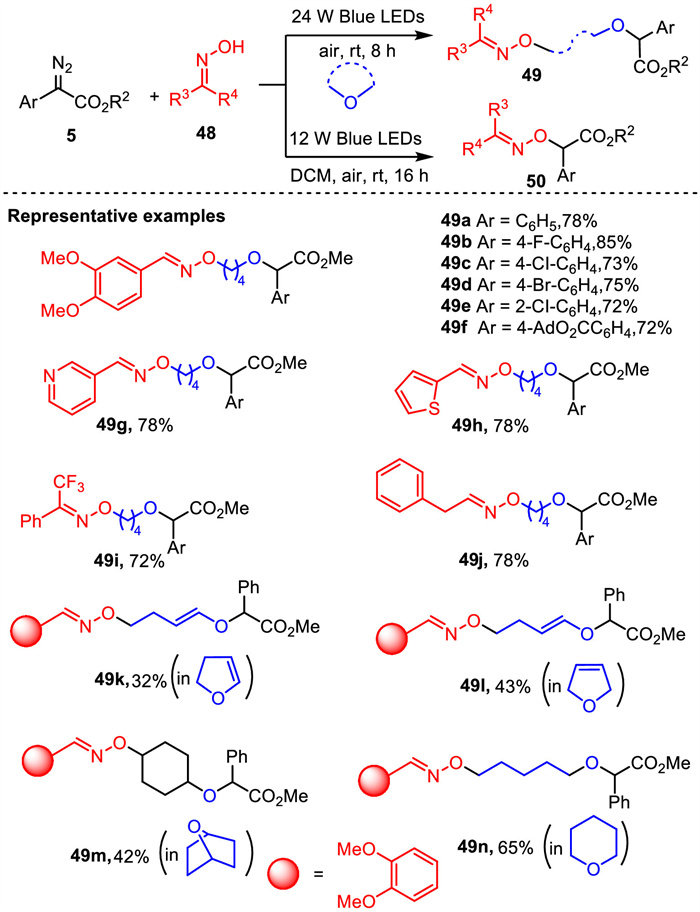
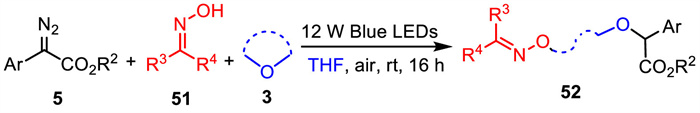
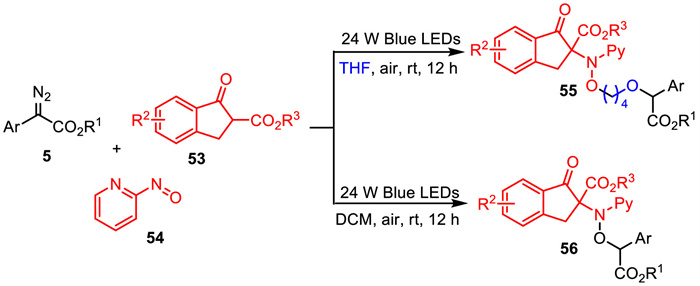
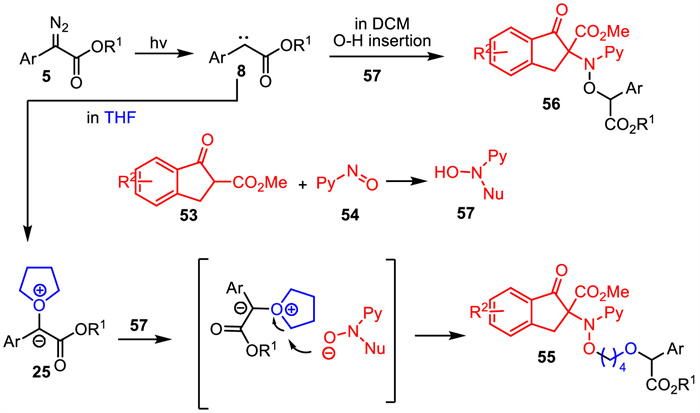
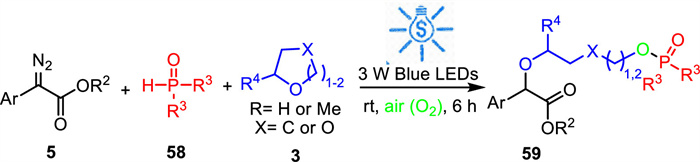
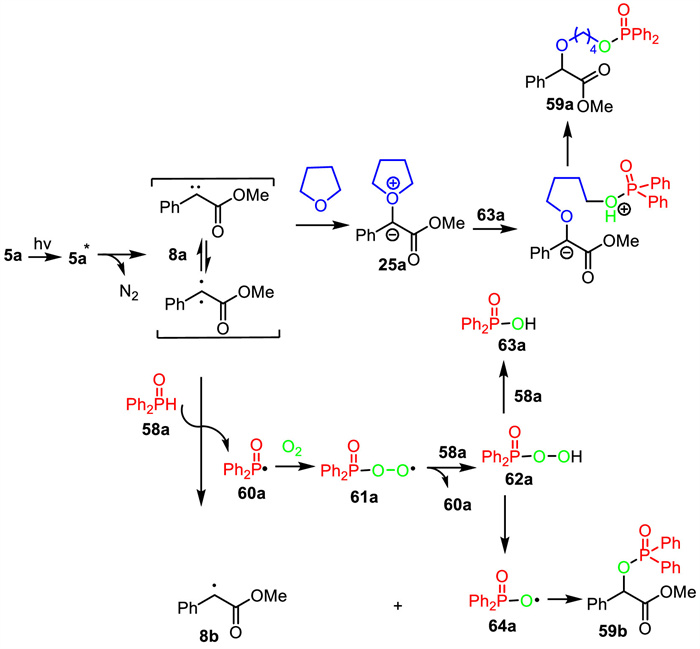
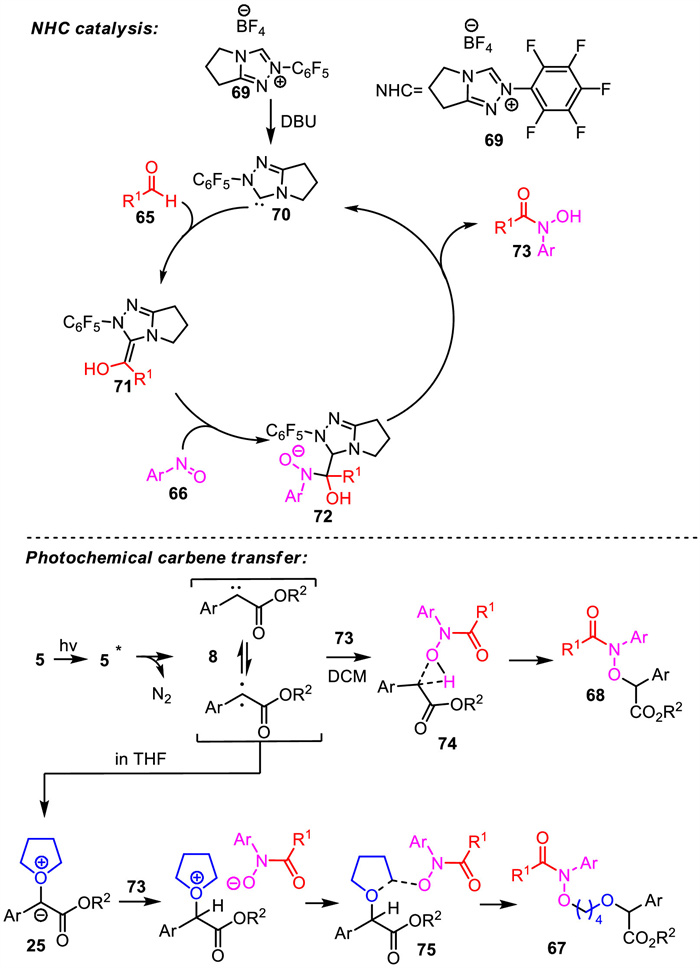
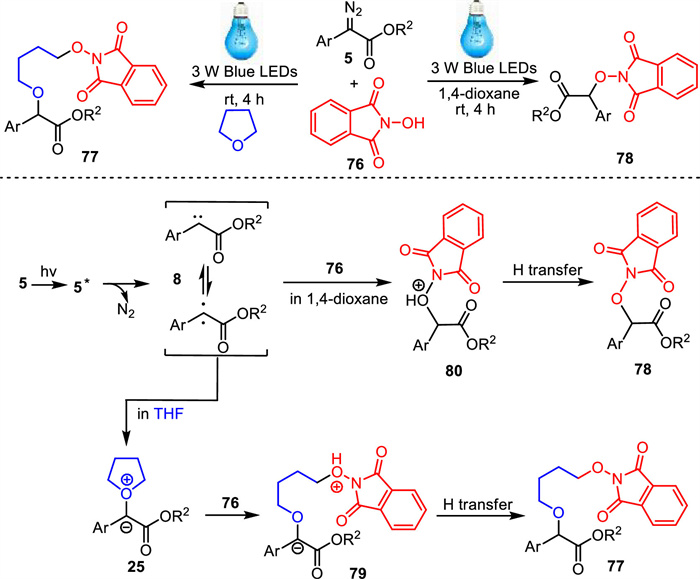
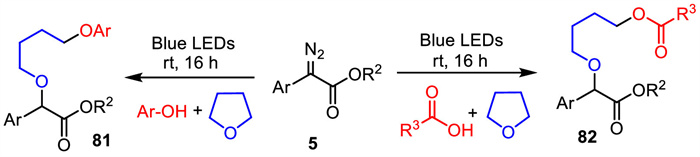
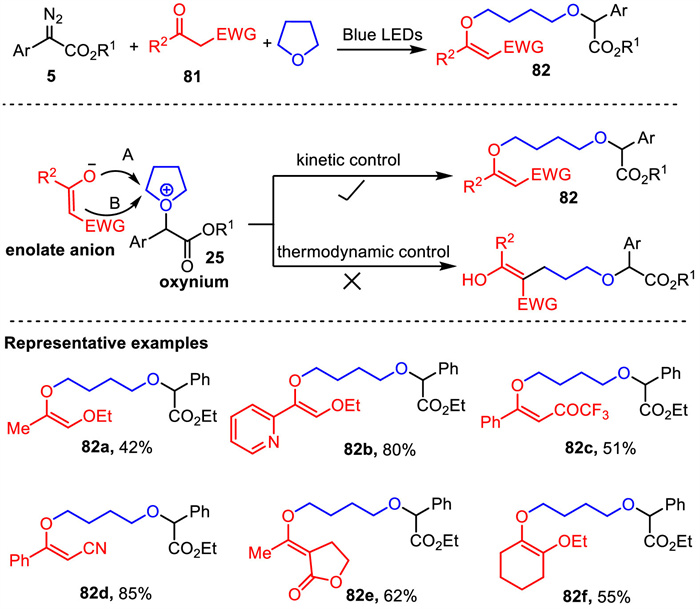
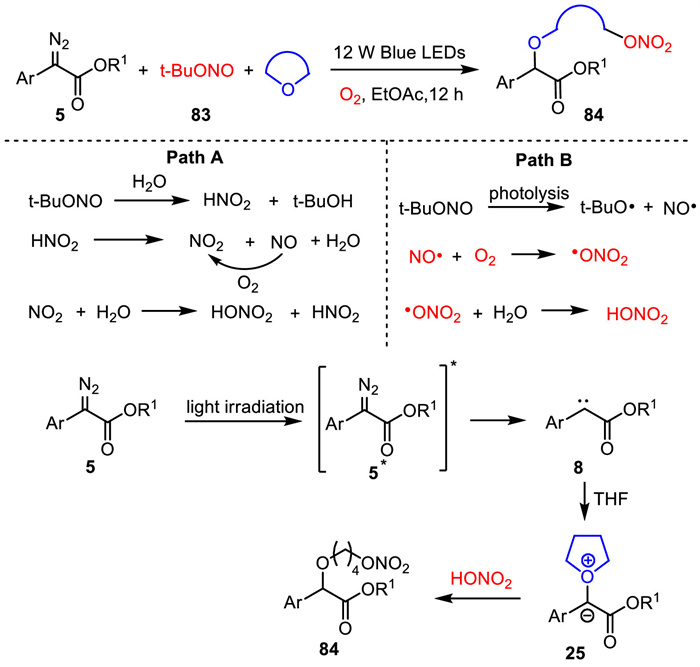
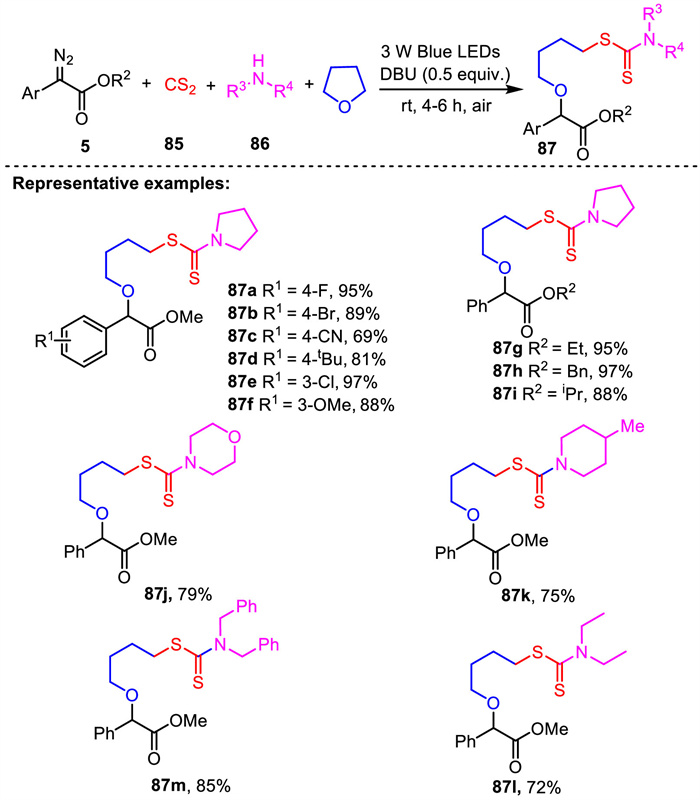
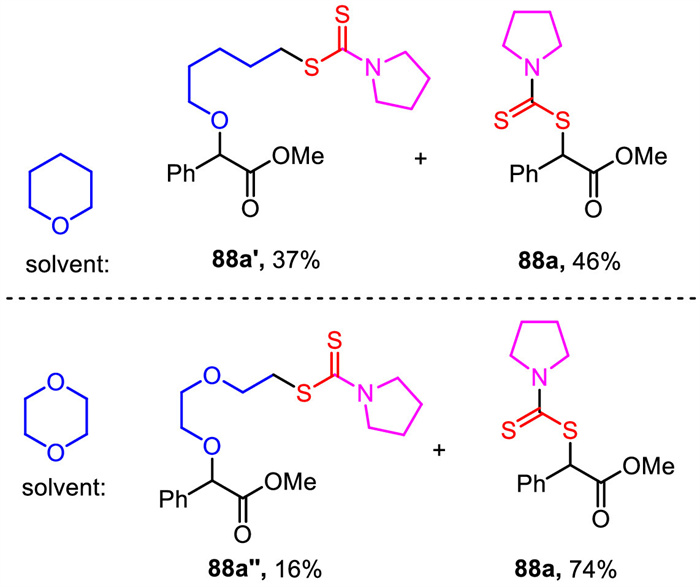
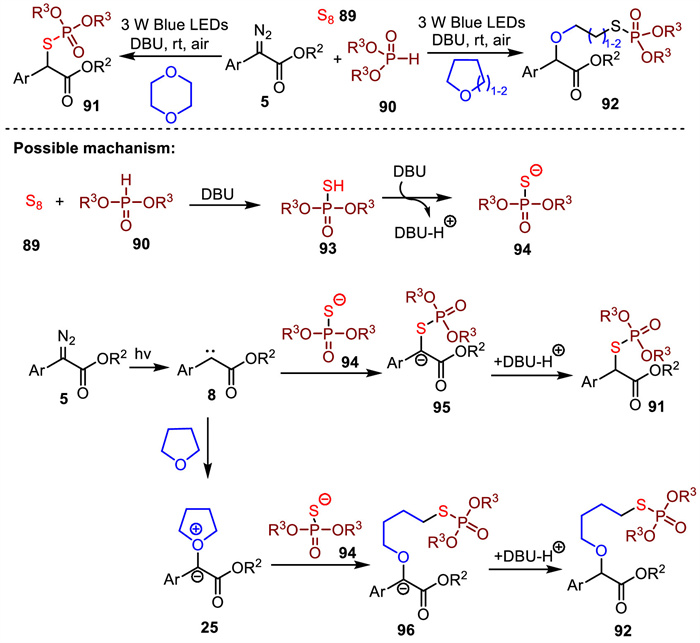
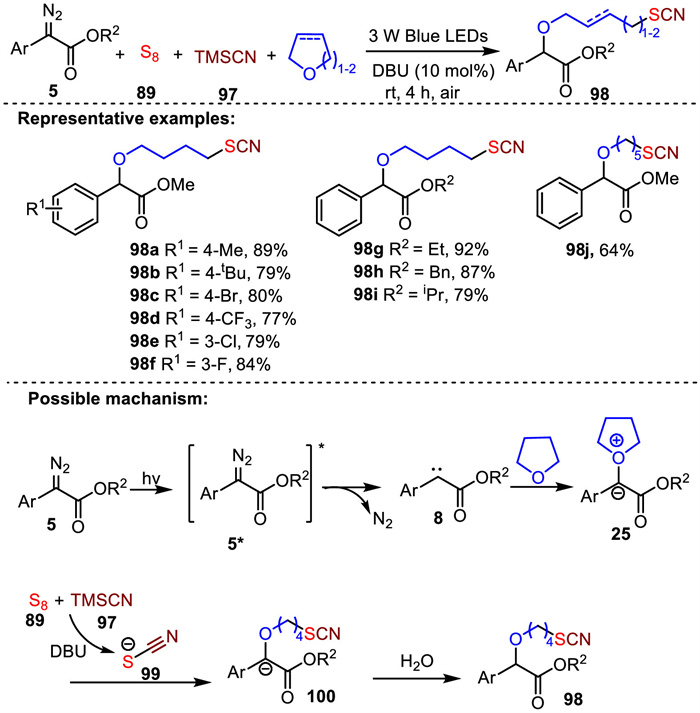
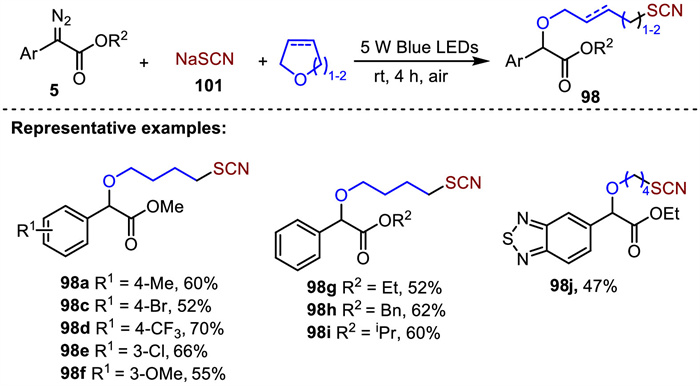
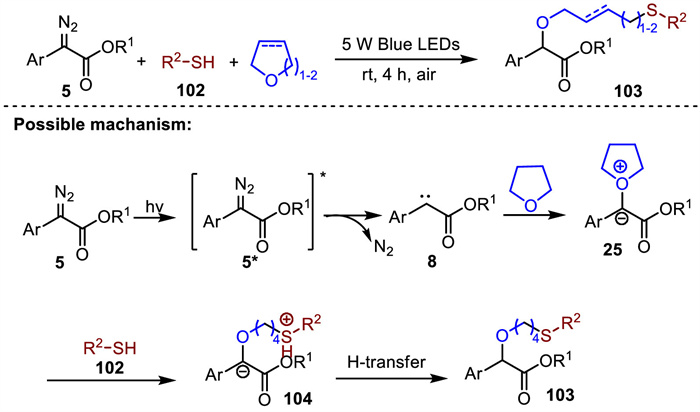
-
[1]
C. Empel, S. Jana, R.M. Koenigs, Molecules 25 (2020) 880.
doi: 10.3390/molecules25040880
-
[2]
S.T. Liu, K.R. Reddy, Chem. Soc. Rev. 28 (1999) 315–322.
-
[3]
T.R. Roose, D.S. Verdoorn, P. Mampuys, et al., Chem. Soc. Rev. 51 (2022) 5842–5877.
doi: 10.1039/d1cs00305d
-
[4]
D. Zhu, L. Chen, H. Fan, et al., Chem. Soc. Rev. 49 (2020) 908–950.
doi: 10.1039/c9cs00542k
-
[5]
M.Y. Huang, S.F. Zhu, Chem. Sci. 12 (2021) 15790–15801.
doi: 10.1039/d1sc03328j
-
[6]
X. Shen, Acc. Chem. Res. 58 (2025) 1519–1533.
doi: 10.1021/acs.accounts.5c00136
-
[7]
Y. Zhang, G. Zhou, S. Liu, X. Shen, Chem. Soc. Rev. 54 (2025) 1870–1904.
doi: 10.1039/d4cs01275e
-
[8]
C. Damiano, P. Sonzini, E. Gallo, Chem. Soc. Rev. 49 (2020) 4867–4905.
doi: 10.1039/d0cs00221f
-
[9]
X. Zhang, L. Li, P. Sivaguru, et al., Chem. Commun. 58 (2022) 13699–13715.
doi: 10.1039/d2cc04845k
-
[10]
D. Qiu, J. Wang, Recent Developments of Diazo Compounds in Organic Synthesis, World Scientific, 2021.
-
[11]
J. Wang, C.M. Che, M.P. Doyle, Transition Metal-Catalyzed Carbene Transformations, John Wiley & Sons, 2021.
-
[12]
Y. He, Z. Huang, K. Wu, et al., Chem. Soc. Rev. 51 (2022) 2759–2852.
doi: 10.1039/d1cs00895a
-
[13]
C. Jing, Q.Q. Cheng, Y. Deng, et al., Org. Lett. 18 (2016) 4550–4553.
doi: 10.1021/acs.orglett.6b02192
-
[14]
C. Qin, H.M.L. Davies, J. Am. Chem. Soc. 135 (2013) 14516–14519.
doi: 10.1021/ja4069003
-
[15]
W. Zhang, S. Chen, Y. Zhang, et al., Angew. Chem. Int. Ed. 64 (2025) e202422020.
-
[16]
D.M. Hodgson, F.Y.T.M. Pierard, P.A. Stupple, Chem. Soc. Rev. 30 (2001) 50–61.
-
[17]
F. Tan, X. Liu, X. Hao, et al., ACS Catal. 6 (2016) 6930–6934.
doi: 10.1021/acscatal.6b02184
-
[18]
S.F. Zhu, B. Xu, G.P. Wang, et al., J. Am. Chem. Soc. 134 (2012) 436–442.
doi: 10.1021/ja2084493
-
[19]
C. Qin, H.M.L. Davies, J. Am. Chem. Soc. 136 (2014) 9792–9796.
doi: 10.1021/ja504797x
-
[20]
Y. Xia, D. Qiu, J.B. Wang, Chem. Rev. 117 (2017) 13810–13889.
doi: 10.1021/acs.chemrev.7b00382
-
[21]
Y. Xia, Y. Zhang, J.B. Wang, ACS Catal. 3 (2013) 2586–2598.
doi: 10.1021/cs4006666
-
[22]
S. Gupta, A. Kundu, S. Ghosh, et al., Green Chem. 25 (2023) 8459–8493.
doi: 10.1039/d3gc03291d
-
[23]
Q. Feng, J. Hao, Y. Hu, et al., Chin. Chem. Lett. 36 (2025) 110582.
-
[24]
H.C. Li, M. Zhang, Q. Lv, et al., Chin. Chem. Lett. 36 (2025) 110579.
-
[25]
Q. Zhao, Q. Zhang, X. Wu, et al., Chin. Chem. Lett. 36 (2025) 110167.
-
[26]
Y. Wang, J. Wang, C. Chen, et al., Chem. Commun. 61 (2025) 10812–10815.
doi: 10.1039/d5cc02744f
-
[27]
Y.H. Lu, C. Wu, J.C. Hou, et al., ACS Catal. 13 (2023) 13071–13076.
doi: 10.1021/acscatal.3c02268
-
[28]
H.T. Ji, K.L. Wang, W.T. Ouyang, et al., Green Chem. 25 (2023) 7983–7987.
doi: 10.1039/d3gc02575f
-
[29]
L.W. Ciszewski, K. Rybicka-Jasinska, D. Gryko, Org. Biomol. Chem. 17 (2019) 432–448.
doi: 10.1039/c8ob02703j
-
[30]
Z. Yang, M.L. Stivanin, I.D. Jurberg, et al., Chem. Soc. Rev. 49 (2020) 6833–6847.
doi: 10.1039/d0cs00224k
-
[31]
W.T. Ouyang, J. Jiang, Y.F. Jiang, et al., Chin. Chem. Lett. 35 (2024) 110038.
-
[32]
H.Y. Song, J. Jiang, C. Wu, et al., Green Chem. 25 (2023) 3292–3296.
doi: 10.1039/d2gc04843d
-
[33]
N. Meng, Y. Lv, Q. Liu, et al., Chin. Chem. Lett. 32 (2021) 258–262.
-
[34]
M. Zhang, G. Nan, X. Zhao, et al., Chin. J. Org. Chem. 42 (2022) 4315–4322.
doi: 10.6023/cjoc202209013
-
[35]
Y.H. Lu, S.Y. Mu, J. Jiang, et al., ChemSusChem 16 (2023) e202300523.
-
[36]
X. Cheng, B.G. Cai, H. Mao, et al., Org. Lett. 23 (2021) 4109–4114.
doi: 10.1021/acs.orglett.1c00979
-
[37]
H.B. Ye, X.Y. Zhou, L. Li, et al., Org. Lett. 24 (2022) 6018–6023.
doi: 10.1021/acs.orglett.2c02313
-
[38]
S. Li, J. Ling, L. Zhou, Org. Lett. 26 (2024) 5220–5225.
doi: 10.1021/acs.orglett.4c01876
-
[39]
I.D. Jurberg, H.M.L. Davies, Chem. Sci. 9 (2018) 5112–5118.
doi: 10.1039/c8sc01165f
-
[40]
T. Xiao, M. Mei, Y. He, L. Zhou, Chem. Commun. 54 (2018) 8865–8868.
doi: 10.1039/c8cc04609c
-
[41]
R. Hommelsheim, Y. Guo, Z. Yang, et al., Angew. Chem. Int. Ed. 58 (2019) 1203–1207.
doi: 10.1002/anie.201811991
-
[42]
B.G. Cai, S.S. Luo, L. Li, et al., CCS Chem. 2 (2020) 2764–2771.
-
[43]
R. Cheng, C. Qi, L. Wang, et al., Chem. Soc. Rev. 49 (2020) 6833–6847.
-
[44]
S. Zhou, B. Cai, C. Hu, et al., Chin. Chem. Lett. 32 (2021) 2577–2581.
-
[45]
Z. Chen, J. Chen, J. Xuan, Chin. Chem. Lett. 33 (2022) 2763–2764.
-
[46]
P. Duan, H. Zhao, J. Yang, et al., Org. Lett. 24 (2022) 608–612.
doi: 10.1021/acs.orglett.1c04048
-
[47]
Y. Guo, G. Zhou, X. Shen, Chin. J. Chem. 42 (2024) 887–902.
doi: 10.1002/cjoc.202300569
-
[48]
G. Zhou, X. Shen, Angew. Chem. Int. Ed. 61 (2022) e202115334.
-
[49]
S. Chen, Y. Zhang, S. Liu, X. Shen, Sci. China Chem. 66 (2023) 3141–3147.
doi: 10.1007/s11426-023-1676-y
-
[50]
R.D.C. Gallo, G. Cariello, T.A.C. Goulart, I.D. Jurberg, Chem. Commun. 59 (2023) 7346–7360.
doi: 10.1039/d3cc00988b
-
[51]
Z. Zhang, V. Gevorgyan, Chem. Rev. 124 (2024) 7214–7261.
doi: 10.1021/acs.chemrev.3c00869
-
[52]
D. Qi, J. Bai, H. Zhang, et al., Green Chem. 24 (2022) 5046–5051.
doi: 10.1039/d2gc01362b
-
[53]
D.K. Jha, S. Acharya, N. Sakkani, et al., Org. Lett. 26 (2024) 172–177.
doi: 10.1021/acs.orglett.3c03805
-
[54]
Y.N. Wang, X. Wang, S.J. Li, Y. Lan, Org. Chem. Front. 9 (2022) 1247–1253.
doi: 10.1039/d1qo01730f
-
[55]
T. Wang, N. Jiao, Acc. Chem. Res. 47 (2014) 1137–1145.
doi: 10.1021/ar400259e
-
[56]
F.F. Fleming, L. Yao, P.C. Ravikumar, et al., J. Med. Chem. 53 (2010) 7902–7917.
doi: 10.1021/jm100762r
-
[57]
J.M. Crance, N. Scaramozzino, A. Jouan, D. Garin, Antiviral Res. 58 (2003) 73–79.
-
[58]
J. Liu, Y. Gong, J. Shi, et al., Eur. J. Med. Chem. 194 (2020) 112244.
-
[59]
W. Yuan, K.J. Szabó, ACS Catal. 6 (2016) 6687–6691.
doi: 10.1021/acscatal.6b01760
-
[60]
F. He, C. Pei, R.M. Koenigs, Chem. Commun. 56 (2020) 599–602.
doi: 10.1039/c9cc08888a
-
[61]
D. Maiti, R. Das, S. Sen, Green Chem. 23 (2021) 8533–8544.
doi: 10.1039/d1gc02797b
-
[62]
S.K. Hota, S. Murarka, Chem. Asian J. (2023) e202301027.
-
[63]
M.L. Stivanin, R.D.C. Gallo, J.P.M. Spadeto, et al., Org. Chem. Front. 9 (2022) 1321–1326.
doi: 10.1039/d1qo01780b
-
[64]
S. Xia, Y. Jian, L. Zhang, et al., RSC Adv. 13 (2023) 14501–14505.
doi: 10.1039/d3ra02407e
-
[65]
Z. Wang, J. Hao, Y. Lv, et al., Asian J. Org. Chem. 11 (2022) e202200484.
-
[66]
B.G. Cai, G.Y. Xu, J. Xuan, Chin. Chem. Lett. 34 (2023) 108335.
-
[67]
S. Fustero, M. Sánchez-Roselló, P. Barrio, A. Simón-Fuentes, Chem. Rev. 111 (2011) 6984–7034.
doi: 10.1021/cr2000459
-
[68]
M.F. Khan, M.M. Alam, G. Verma, et al., Eur. J. Med. Chem. 120 (2016) 170–201.
-
[69]
V. Kumar, K. Kaur, G.K. Gupta, A.K. Sharma, Eur. J. Med. Chem. 69 (2013) 735–753.
-
[70]
H. Ding, Z. Wang, C. Qu, et al., Org. Chem. Front. 9 (2022) 5530–5535.
doi: 10.1039/d2qo01082h
-
[71]
J. Klankermayer, S. Wesselbaum, K. Beydoun, W. Leitner, Angew. Chem. Int. Ed. 55 (2016) 7296–7343.
doi: 10.1002/anie.201507458
-
[72]
S.S. Yan, Q. Fu, L.L. Liao, et al., Coord. Chem. Rev. 374 (2018) 439–463.
-
[73]
R. Cheng, C. Qi, L. Wang, et al., Green Chem. 22 (2020) 4890–4895.
doi: 10.1039/d0gc00910e
-
[74]
B. Rong, G. Xu, H. Yan, et al., Chem. Commun. 57 (2021) 7272–7275.
doi: 10.1039/d1cc02422a
-
[75]
M. Murakami, N. Ishida, Chem. Rev. 121 (2021) 264–299.
doi: 10.1021/acs.chemrev.0c00569
-
[76]
Q. Li, B.G. Cai, L. Li, J. Xuan, Org. Lett. 23 (2021) 6951–6955.
doi: 10.1021/acs.orglett.1c02555
-
[77]
M.L. Stivanin, M. Duarte, L.P.M.O. Leão, et al., J. Org. Chem. 86 (2021) 17528–17532.
doi: 10.1021/acs.joc.1c02411
-
[78]
B.-G. Cai, Q. Li, Q. Zhang, et al., Org. Chem. Front. 8 (2021) 5982–5987.
doi: 10.1039/d1qo01102b
-
[79]
C. Qu, J. Hao, H. Ding, et al., J. Org. Chem. 87 (2022) 12921–12931.
doi: 10.1021/acs.joc.2c01499
-
[80]
S. Bertrand, J.J. Helesbeux, G. Larcher, O. Duval, Mini-Rev. Med. Chem. 13 (2013) 1311–1326.
doi: 10.2174/13895575113139990007
-
[81]
A. Citarella, D. Moi, L. Pinzi, et al., ACS Omega 6 (2021) 21843–21849.
doi: 10.1021/acsomega.1c03628
-
[82]
B.G. Cai, Li Q, C. Empel, et al., ACS Catal. 12 (2022) 11129–11136.
doi: 10.1021/acscatal.2c02875
-
[83]
R. Liu, S. Fu, X. Chu, et al., Chin. J. Org. Chem. 42 (2022) 2462–2470.
doi: 10.6023/cjoc202204014
-
[84]
K. Zhu, W.J. Yu, X. Zhou, et al., Chem. Commun. 59 (2023) 12605–12608.
doi: 10.1039/d3cc03317a
-
[85]
P. Li, X. Jia, Synthesis (2018) 711–722.
-
[86]
R. Bag, D. Sar, T. Punniyamurthy, ACS Omega 2 (2017) 6278–6290.
doi: 10.1021/acsomega.7b01111
-
[87]
B.A. Mir, S. Rajamanickam, P. Begum, B.K. Patel, Eur. J. Org. Chem. (2020) 2617–2625.
doi: 10.1002/ejoc.202000149
-
[88]
Y. Lv, J. Hao, J. Huang, et al., Chem. Commun. 60 (2024) 9801–9804.
doi: 10.1039/d4cc03272a
-
[89]
S. Dutta, H. Abe, S. Aoyagi, et al., J. Am. Chem. Soc. 127 (2005) 15004–15005.
doi: 10.1021/ja053735i
-
[90]
M. Burow, A. Bergner, J. Gershenzon, U. Wittstock, Plant Mol. Biol. 63 (2006) 49–61.
doi: 10.1007/s11103-006-9071-5
-
[91]
R. Ding, S. Shi, C. Ma, et al., Chin. J. Org. Chem. 44 (2024) 2216–2222.
doi: 10.6023/cjoc202403029
-
[92]
X.M. Chen, L. Song, J. Pan, et al., Chin. Chem. Lett. 35 (2024) 110112.
-
[93]
X. Chen, J. Huang, J. Pan, et al., Org. Lett. 26 (2024) 3883–3888.
doi: 10.1021/acs.orglett.4c01038
-
[94]
J. Li, C. Chen, X. Li, et al., Chin. Chem. Lett. 35 (2024) 109732.
-
[95]
J. Hao, Y. Lv, S. Tian, et al., Chin. Chem. Lett. 35 (2024) 109513.
-
[96]
C. Wu, S. Wu, Q. Huang, et al., Chin. Chem. Lett. 36 (2025) 110250.
-
[97]
Y. Lv, R. Liu, H. Ding, et al., Org. Chem. Front. 9 (2022) 3486–3492.
doi: 10.1039/d2qo00311b
-
[98]
C. Qu, R. Liu, Z. Wang, et al., Green Chem. 24 (2022) 4915–4920.
doi: 10.1039/d2gc00903j
-
[99]
Z. Wang, R. Liu, C. Qu, et al., Org. Chem. Front. 9 (2022) 3565–3570.
doi: 10.1039/d2qo00539e
-
[100]
Z. Wang, N. Meng, Y. Lv, et al., Chin. Chem. Lett. 34 (2023) 107599.
-
[101]
Y. Lv, Z. Wang, J. Hao, et al., Asian J. Org. Chem. 12 (2023) e202300290.
-
[102]
Y. Lv, H. Ding, J. You, et al., Chin. Chem. Lett. 35 (2024) 109107.

 Login In
Login In







 DownLoad:
DownLoad: