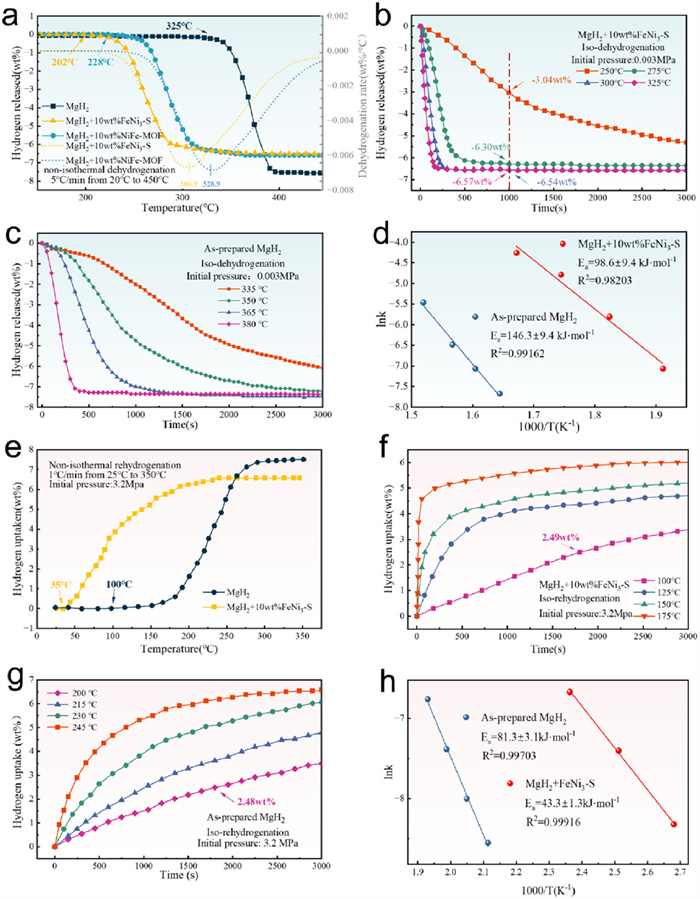
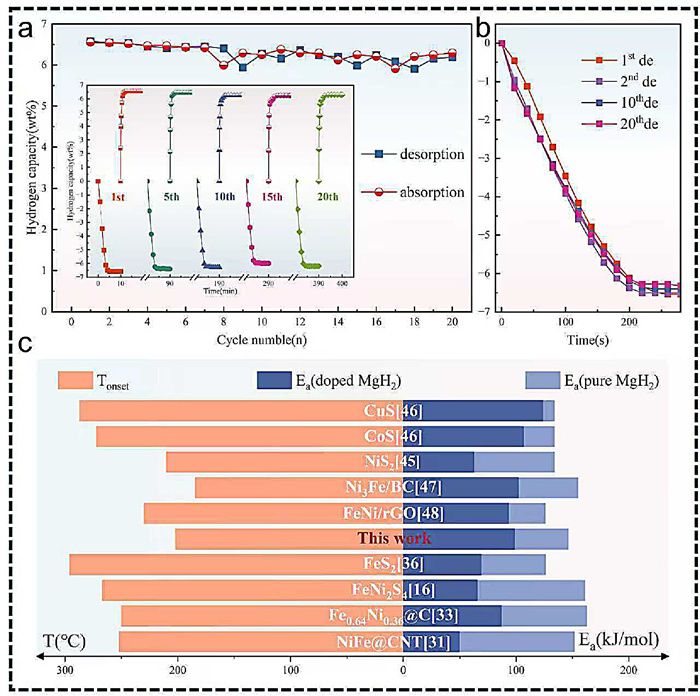
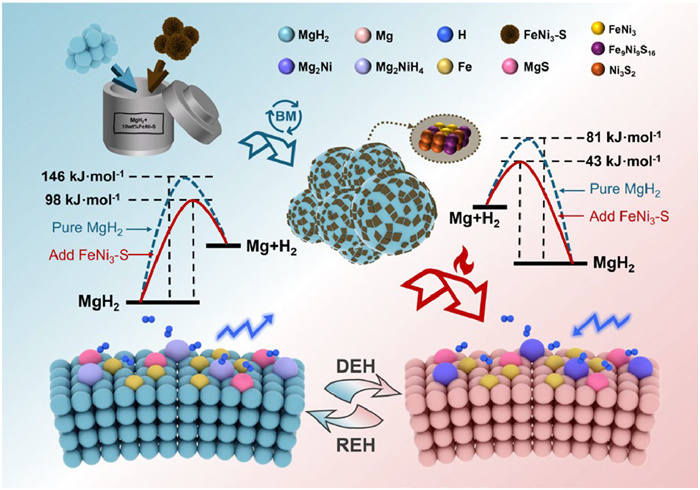
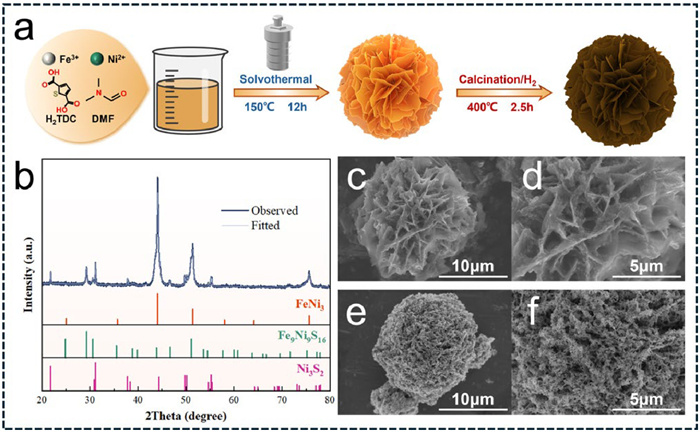
-
[1]
J.O. Abe, A.P.I. Popoola, E. Ajenifuja, et al., Int. J. Hydrog. Energy 44 (2019) 15072–15086.
-
[2]
M.R. Usman, Renew. Sustain. Energy Rev. 167 (2022) 112743.
-
[3]
E.H. Abdechafik, H.A. Ousaleh, S. Mehmood, et al., Int. J. Hydrog. Energy 52 (2024) 1182–1193.
-
[4]
O. Faye, J. Szpunar, U. Eduok, Int. J. Hydrog. Energy 47 (2022) 13771–13802.
-
[5]
Y. Wang, Y. Xue, A. Züttel, Chem. Soc. Rev. 53 (2024) 972–1003.
doi: 10.1039/d3cs00706e
-
[6]
X. Zhang, Y. Liu, X. Zhang, et al., Mater. Today Nano 9 (2020) 100064.
-
[7]
L. Ren, Y. Li, N. Zhang, et al., Nano Micro Lett. 15 (2023) 93.
-
[8]
C. Zhou, Y. Peng, Q. Zhang, J. Mater. Sci. Technol. 50 (2020) 178–183.
-
[9]
Y. Shang, C. Pistidda, G. Gizer, et al., J. Magn. Alloy. 9 (2021) 1837–1860.
-
[10]
V.V. Berezovets, R.V. Denys, I.Y. Zavaliy, et al., Int. J. Hydrog. Energy 47 (2022) 7289–7298.
-
[11]
Y. Zhang, G. Wu, J. Gu, et al., Rare Met. 43 (2024) 3260–3272.
doi: 10.1007/s12598-024-02627-7
-
[12]
J. Zhang, X. Ding, R. Chen, et al., J. Power Sources 548 (2022) 232037.
-
[13]
Y. Fu, Z. Ding, S. Ren, et al., Int. J. Hydrog. Energy 45 (2020) 28154–28162.
-
[14]
H. Huang, T. Xu, J. Chen, et al., Chem. Eng. J. 483 (2024) 149434.
-
[15]
S. Li, L. Zhang, F. Wu, et al., Chin. Chem. Lett. 36 (2025) 109566.
-
[16]
Y. Fu, L. Zhang, Y. Li, et al., J. Magn. Alloys. 11 (2023) 2927–2938.
-
[17]
X. Xie, B. Zhang, H. Kimura, et al., Chem. Eng. J. 464 (2023) 142630.
-
[18]
N.S. Norberg, T.S. Arthur, S.J. Fredrick, et al., J. Am. Chem. Soc. 133 (2011) 10679–10681.
doi: 10.1021/ja201791y
-
[19]
X. Li, Y. Fu, Y. Xie, et al., Int. J. Hydrog. Energy 46 (2021) 33186–33196.
-
[20]
X. Yang, Q. Hou, L. Yu, et al., Dalton Trans. 50 (2021) 1797–1807.
doi: 10.1039/d0dt03627g
-
[21]
M. Song, L. Zhang, Z. Yao, et al., Inorg. Chem. Front. 9 (2022) 3874–3884.
doi: 10.1039/d2qi00863g
-
[22]
P.K. Soni, A. Bhatnagar, M.A. Shaz, Int. J. Hydrogen Energy 48 (2023) 17970–17982.
-
[23]
D. Pukazhselvan, N. Nasani, P. Correia, et al., J. Power Sources 362 (2017) 174–183.
-
[24]
D. Pukazhselvan, K.S. Sandhya, D. Ramasamy, et al., ChemPhysChem 21 (2020) 1195–1201.
doi: 10.1002/cphc.202000031
-
[25]
B. Liu, B. Zhang, X. Chen, et al., Mater. Today Nano 17 (2022) 100168.
-
[26]
L. Li, G. Jiang, H. Tian, et al., Int. J. Hydrog. Energy 42 (2017) 28464–28472.
-
[27]
Y. Meng, J. Zhang, S. Ju, et al., J. Mater. Chem. A 11 (2023) 9762–9771.
doi: 10.1039/d3ta01029e
-
[28]
Z. Wang, Z. Ren, N. Jian, et al., J. Mater. Chem. A 6 (2018) 16177–16185.
doi: 10.1039/c8ta05437a
-
[29]
S. Guo, Z. Yu, Y. Li, et al., J. Alloy. Compd. 976 (2024) 173035.
-
[30]
L. Zhang, H. Yu, Z. Lu, et al. Chin. J. Chem. Eng. 43 (2022) 343–352.
-
[31]
Y. Fu, Z. Yu, S. Guo, et al., Chem. Eng. J. 458 (2023) 141337.
-
[32]
M. Pozzo, D. Alfè, Int. J. Hydrog. Energy 34 (2009) 1922–1930.
-
[33]
Z. Ding, Y. Fu, L. Zhang, et al., J. Alloy. Compd. 843 (2020) 156035.
-
[34]
W. He, F. Wang, Y. Gao, et al., Sustain. Energy Fuels 6 (2022) 3852–3857.
doi: 10.1039/d2se00886f
-
[35]
J. Mei, Y. Deng, X. Cheng, et al., Chin. Chem. Lett. 35 (2024) 108900.
-
[36]
W. Zhang, G. Xu, Y. Cheng, et al., Dalton Trans. 47 (2018) 5217–5225.
doi: 10.1039/c7dt04665k
-
[37]
W. Zhang, Y. Cheng, D. Han, et al., Energy 93 (2015) 625–630.
-
[38]
L. Zeng, Z. Lan, B. Li, et al., J. Magn. Alloy. 10 (2022) 3628–3640.
-
[39]
D. Zhou, K. Cui, Z. Zhou, et al., Int. J. Hydrog. Energy 46 (2021) 34369–34380.
-
[40]
Y. Qian, F. Zhang, S. Zhao, et al., Nano Energy 111 (2023) 108415.
-
[41]
Y. Chen, R. Zhang, L. Jiao, et al., Coord. Chem. Rev. 362 (2018) 1–23.
-
[42]
S. Zhang, Z. Huang, T.T. Isimjan, et al., Appl. Catal. B Environ. 343 (2024) 123448.
-
[43]
L. Ren, Y. Li, Z. Li, et al., Nano Micro Lett. 16 (2024) 160.
-
[44]
L. Zhang, L. Ji, Z. Yao, et al., Int. J. Hydrog. Energy 44 (2019) 21955–21964.
-
[45]
P. Wang, Z. Wang, Z. Tian, et al., Renew. Energy 160 (2020) 409–417.
-
[46]
P. Wang, Z. Tian, Z. Wang, et al., Int. J. Hydrog. Energy 46 (2021) 27107-27118.
-
[47]
Q. Hou, J. Zhang, Z. Zheng, et al., Dalton Trans. 51 (2022) 14960-14969.
doi: 10.1039/d2dt02425j
-
[48]
L. Ji, L. Zhang, X. Yang, et al., Dalton Trans. 49 (2020) 4146-4154.
doi: 10.1039/d0dt00230e
-
[49]
T. Zhong, T. Xu, L. Zhang, et al., J. Magn. Alloy. 13 (2025) 148-160.

 Login In
Login In






 DownLoad:
DownLoad: