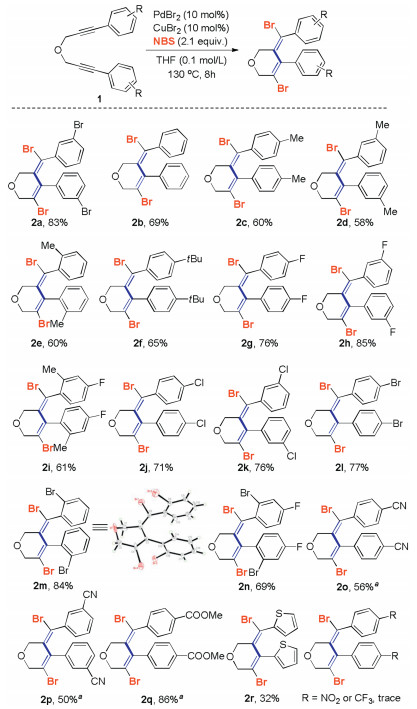
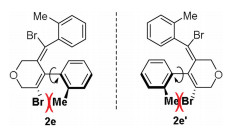
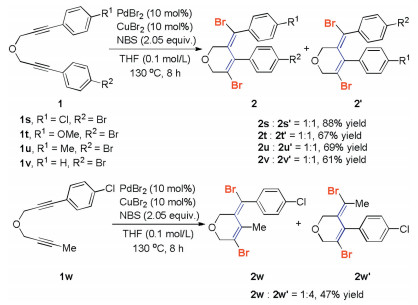
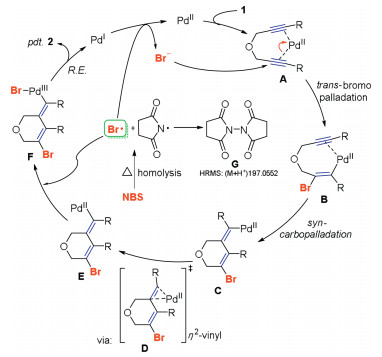
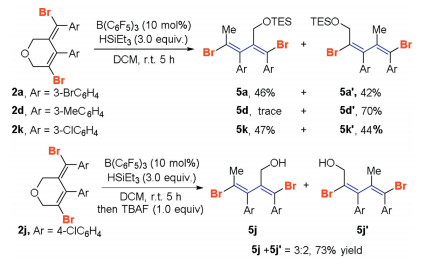
-
[1]
(a) I.G. Stara, I. Stary, Acc. Chem. Res. 53 (2020) 144-158;
(b) L. Chung, C. Wong, Chem. Eur. 25 (2019) 2889-2897;
(c) Y. Hu, M. Bai, Y. Yang, Q. Zhou, Org. Chem. Front. 4 (2017) 2256-2275;
(d) C. Shu, L. Li, T. Tan, D. Yuan, L. Ye, Sci. Bull. 62 (2017) 352-357;
(e) T. Müller, Metal Catalyzed Cascade Reactions, Vol. 19, Springer, Berlin/Heidelberg, 2006, pp. 149-205.
-
[2]
(a) D. Rodríguez, M. Martínez-Esperón, A. Navarro-Vázquez, et al., J. Org. Chem. 69 (2004) 3842-3848;
(b) R.C. Burell, K.J. Daoust, A.Z. Bradley, K.J. Dirico, P.R. Johnson, J. Am. Chem. Soc. 118 (1996) 4218-4219;
(c) D. Rodríguez, A. Navarro, L. Castedo, D. Domínguez, C. Saá, Org. Lett. 2 (2000) 1497-1500;
(d) A.G. Myers, E.Y. Kuo, N.S. Finney, J. Am. Chem. Soc. 111 (1989) 8057-8059.
-
[3]
(a) M. Huang, C. Zhu, C. He, et al., Org. Chem. Front. 5 (2018) 1643-1650;
(b) M. Huang, Y. Zhu, W. Hao, et al., Adv. Synth. Catal. 359 (2017) 2229-2234.
-
[4]
S. Saito, Y. Yamamoto, Chem. Rev. 100 (2000) 2901-2915.
doi: 10.1021/cr990281x
-
[5]
(a) J. Zhao, C.O. Hughes, F.D. Toste, J. Am. Chem. Soc. 128 (2006) 7436-7437;
(b) C. Oh, A. Kim, W. Park, D. Park, N. Kim, Synlett 17 (2006) 2781-2784.
-
[6]
(a) Y. Chen, M. Chen, Y. Liu, Angew. Chem. Int. Ed. 51 (2012) 6493-6497;
(b) Q. Xiao, H. Zhu, G. Li, Z. Chen, Adv. Synth. Catal. 356 (2014) 3809-3815;
(c) B.M. Trost, T. Michael, T. Rudd, J. Am. Chem. Soc. 127 (2005) 4763-4776.
-
[7]
(a) J. Zhang, H. Wang, S. Ren, W. Zhang, Y. Liu, Org. Lett. 17 (2005) 2920-2923;
(b) W. Hui, Y. Zhou, Y. Dong, et al., Green Energy Environ. 4 (2019) 53-59;
(c) J. Zhang, H. Zhang, D. Shi, H. Jin, Y. Liu, Eur. J. Org. Chem. 33 (2016) 5545-5558.
-
[8]
(a) D. Leboeuf, A. Simonneau, C. Aubert, et al., Angew. Chem. Int. Ed. 50 (2011) 6868-6871;
(b) J. Lian, P. Chen, Y. Lin, H. Ting, R.S. Liu, J. Am. Chem. Soc. 128 (2006) 11372-11373.
-
[9]
Y. Li, K.J. Jardine, R. Tan, D. Song, V.M. Dong, Angew. Chem. Int. Ed. 48 (2009) 9690-9692.
doi: 10.1002/anie.200905478
-
[10]
(a) Z. Chen, M. Zeng, J. Yuan, Q. Yang, Y. Peng, Org. Lett. 14 (2012) 3588-3591;
(b) Z. Chen, X. Jia, C. Ye, G. Qiu, J. Wu, Chem. Asian J. 9 (2014) 126-130;
(c) J. Tan, Z. Wang, J. Yuan, Y. Peng, Z. Chen, Adv. Synth. Catal. 361 (2019) 1295-1300;
(d) H. Zhu, Z. Chen, Org. Lett. 18 (2016) 488-491;
(e) Z. Chen, X. Jia, J. Huang, J. Yuan, J. Org. Chem. 79 (2014) 10674-10681.
-
[11]
K. Strom, A. Impastato, K. Moy, A. Landreth, J. Snyder, Org. Lett. 17 (2015) 2126-2129.
-
[12]
(a) K. Albert, G. Schlotterbeck, U. Braumann, et al., Angew. Chem. Int. Ed. 34 (1995) 1014-1016;
(b) B. Vaz, R. Alvarez, A.R. de Lera, J. Org. Chem. 67 (2002) 5040-5043.
-
[13]
(a) J. Li, Y. Liang, Y. Xie, J. Org. Chem. 69 (2004) 8125-8127;
(b) Y. Wen, L. Huang, H. Jiang, H. Chen, J. Org. Chem. 77 (2012) 2029-2034.
-
[14]
S. Sarez-Pantiga, D. Palomas, E. Rubio, J. Gonzalez, Angew. Chem. Int. Ed. 48 (2009) 7857-7861.
doi: 10.1002/anie.200902989
-
[15]
(a) G. Zhu, Z. Zhang, J. Org. Chem. 70 (2005) 3339-3341;
(b) H. Jiang, C. Qiao, W. Liu, Chem. Eur. J. 16 (2010) 10968-10970;
(c) S. Tang, Q. Yu, P. Peng, et al., Org. Lett. 9 (2007) 3413-3416;
(d) D. Chen, X. Chen, Z. Lu, et al., Adv. Synth. Catal. 353 (2011) 1474-1478;
(e) J. Li, S. Tang, Y. Xie, J. Org. Chem. 70 (2005) 477-479.
-
[16]
(a) L. Bai, Y. Yuan, J. Liu, et al., Angew. Chem. Int. Ed. 55 (2016) 6946-6950;
(b) H. Yoon, M. Rçlz, F. Landau, M. Lautens, Angew. Chem. Int. Ed. 56 (2017) 10920-10923;
(c) J. Wallbaum, R. Neufeld, D. Stalke, D.B. Werz, Angew. Chem. Int. Ed. 52 (2013) 13243-13246;
(d) B. Monks, S. Cook, J. Am. Chem. Soc. 134 (2012) 15297-15300;
(e) S. Blouin, R. Pertschi, A. Schoenfelder, J. Suffert, G. Blond, Adv. Synth. Catal. 360 (2018) 2166-2171;
(f) S. Blouin, V. Gandon, G. Blond, J. Suffert, Angew. Chem. Int. Ed. 55 (2016) 7208-7211;
(g) J. Petrignet, A. Boudhar, G. Blond, J. Suffert, Angew. Chem. Int. Ed. 50 (2011) 3285-3289.
-
[17]
(a) A. Reding, P. Jones, D.B. Werz, Angew. Chem. Int. Ed. 57 (2018) 10610-10614;
(b) M. Pawliczek, T. Schneider, C. Maass, D. Stalke, D.B. Werz, Angew. Chem. Int. Ed. 54 (2015) 4119-4123;
(c) T. Sperger, C. Le, M. Lautens, F. Schoenebeck, Chem. Sci. 8 (2017) 2914-2922;
(d) C. Le, P. Menzies, D. Petrone, M. Lautens, Angew. Chem. Int. Ed. 54 (2015) 254-257.
-
[18]
Z. Liu, M. Zhang, X. Wang, J. Am. Chem. Soc. 142 (2020) 581-588.
doi: 10.1021/jacs.9b11909

 Login In
Login In







 DownLoad:
DownLoad: