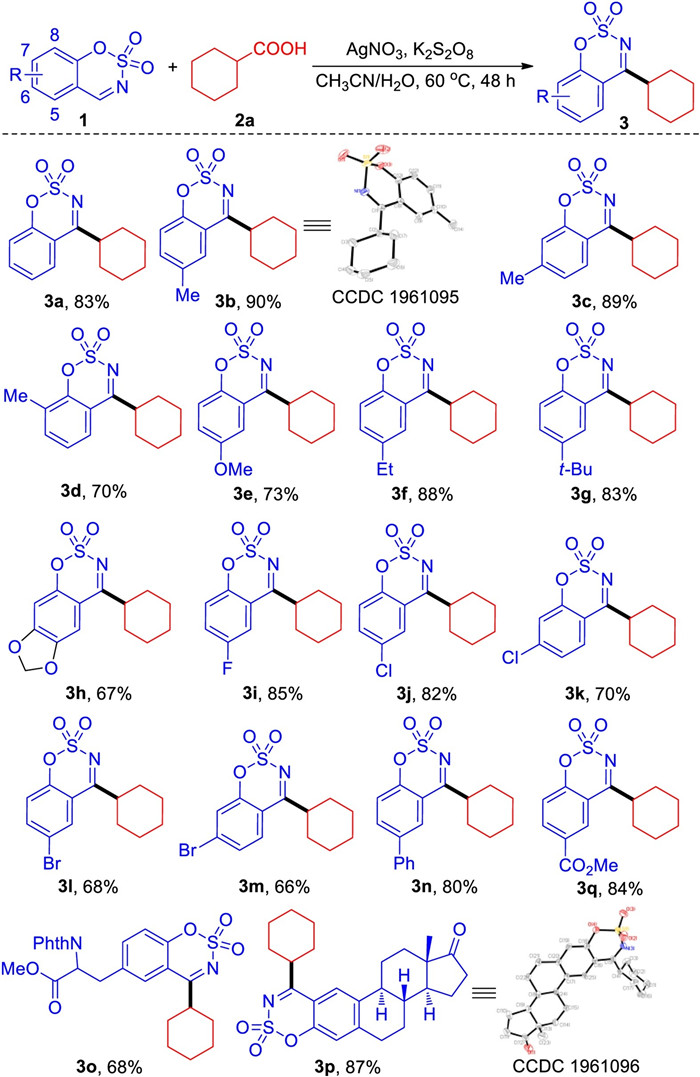
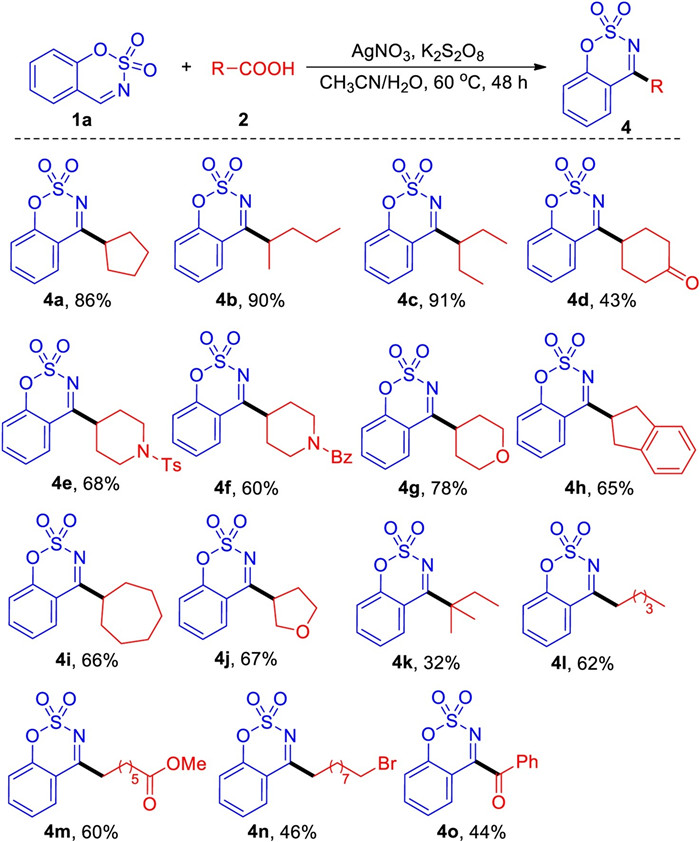
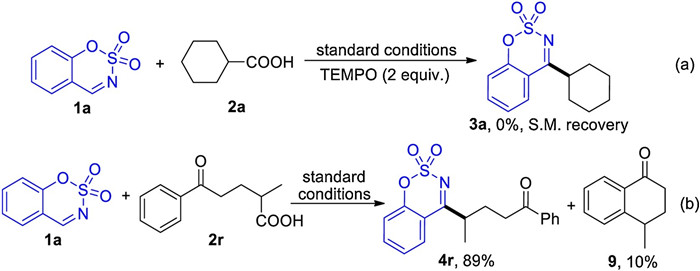
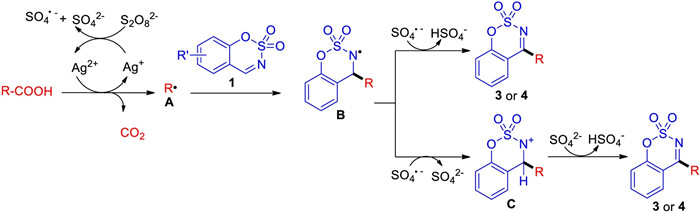
-
[1]
(a) K.C. Majumdar, S. Mondal, Chem. Rev. 111 (2011) 7749-7773;
(b) S.J. Williams, Expert Opin. Ther. Pat. 23 (2013) 79-98;
(c) S. Debnath, S. Mondal, Eur. J. Org. Chem. 2018 (2018) 933-956.
-
[2]
(a) Y. Luo, H.B. Hepburn, N. Chotsaeng, H.W. Lam, Angew. Chem. Int. Ed. 51 (2012) 8309-8313;
(b) J.I. Martinez, J.J. Smith, H.B. Hepburn, H.W. Lam, Angew. Chem. Int. Ed. 55 (2016) 1108-1112;
(c) J. Park, S. Choi, Y. Lee, S.H. Cho, Org. Lett. 19 (2017) 4054-4057.
-
[3]
(a) H. Wang, T. Jiang, M.H. Xu, J. Am. Chem. Soc. 135 (2013) 971-974;
(b) M. Quan, L. Tang, J. Shen, G. Yang, W. Zhang, Chem. Commun. 53 (2017) 609-612;
(c) B. Zhou, K. Li, C. Jiang, Y. Lu, T. Hayashi, Adv. Synth. Catal. 359 (2017) 1969-1975;
(d) W. Sun, H. Gu, X. Lin, J. Org. Chem. 83 (2018) 4034-4043.
-
[4]
(a) L.D. Munck, A. Monleón, C. Vila, J.R. Pedro, Adv. Synth. Catal. 359 (2017) 1582-1587;
(b) Y.L. Li, J.X. Liu, X.P. Chen, et al., Adv. Synth. Catal. 362 (2020) 3202-3207.
-
[5]
(a) Y. Luo, A.J. Carnell, H.W. Lam, Angew. Chem. Int. Ed. 51 (2012) 6762-6766;
(b) S.S. Zhang, T.J. Hu, M.Y. Li, et al., Angew. Chem. Int. Ed. 58 (2019) 3387-3391.
-
[6]
(a) H.X. Zhang, J. Nie, H. Cai, J.A. Ma, Org. Lett. 16 (2014) 2542-2545;
(b) C.M. Jia, H.X. Zhang, J. Nie, J.A. Ma, J. Org. Chem. 81 (2016) 8561-8569;
(c) X.Y. Cui, H.X. Duan, Y. Zhang, Y.Q. Wang, Chem. Asian J. 11 (2016) 3118-3125;
(d) J. Zhao, Y. Li, L.Y. Chen, X. Ren, J. Org. Chem. 84 (2019) 5099-5108.
-
[7]
(a) S.G. Lee, S.G. Kim, RSC Adv. 7 (2017) 34283-34286;
(b) C. Vila, A. Tortosa, G. Blay, et al., New J. Chem. 43 (2019) 130-134.
-
[8]
(a) Y. Liu, T.R. Kang, Q.Z. Liu, et al., Org. Lett. 15 (2013) 6090-6093;
(b) J. Wang, F. Li, Q. Shen, et al., Synthesis 48 (2016) 441-447;
(c) B. Mao, W. Shi, J. Liao, et al., Org. Lett. 19 (2017) 6340-6343;
(d) K. Spielmann, A. Lee, et al., Org. Lett. 20 (2018) 1444-1447;
(e) J. Zhou, H. Zhang, X.L. Chen, et al., J. Org. Chem. 84 (2019) 9179-9187;
(f) J.U. Park, H.I. Ahn, H.J. Cho, et al., Adv. Synth. Catal. 362 (2020) 1836-1840;
(g) C.C. Wang, Z.W. Ma, Y.L. Qu, et al., Chem. Asian J. 15 (2020) 560-563;
(h) J.A. Xiao, X.L. Cheng, H. Peng, et al., Sci. China Chem. 63 (2020) 785-791;
(i) H.I. Ahn, J.U. Park, Z. Xuan, J.H. Kim, Org. Biomol. Chem. 18 (2020) 9826-9830.
-
[9]
(a) Z. Yang, H. Yu, L. Zhang, et al., Chem. Asian J. 9 (2014) 313-318;
(b) A.J. Xia, T.R. Kang, L. He, et al., Angew. Chem. Int. Ed. 55 (2016) 1441-1444.
-
[10]
(a) X. Qian, J. Han, L. Wang, RSC Adv. 6 (2016) 89234-89237;
(b) F. Li, J. Wang, W. Pei, et al., Tetrahedron Lett. 59 (2018) 3010-3014.
-
[11]
K. Parthasarathy, A.R. Azcargorta, Y. Cheng, C. Bolm, Org. Lett. 16(2014) 2538-2541.
doi: 10.1021/ol500918t
-
[12]
(a) B.N. Lai, J.F. Qiu, H.X. Zhang, J. Nie, J.A. Ma, Org. Lett. 18 (2016) 520-523;
(b) L.D. Munck, C. Vila, M.C. Muñoz, J.R. Pedro, Chem. Eur. J. 22 (2016) 17590-17594;
(c) J. Liu, X. Tong, M. Chen, J. Org. Chem. 85 (2020) 5193-5202;
(d) K.P. Jethava, J. Fine, Y. Chen, A. Hossain, G. Chopra, Org. Lett. 22 (2020) 8480-8486;
(e) Y. Zhao, X.Q. Wang, Y.J. Yu, Y.G. Zhou, J. Org. Chem. 86 (2021) 1262-1272.
-
[13]
J. Wang, X. Liu, Z. Wu, et al., Chem. Commun. 57(2021) 1506-1509.
doi: 10.1039/D0CC07181A
-
[14]
F. Minisci, R. Bernardi, F. Bertini, R. Galli, M. Perchinummo, Tetrahedron 27(1971) 3575-3579.
doi: 10.1016/S0040-4020(01)97768-3
-
[15]
(a) C.J. Li, X. Bi, Silver Catalysis in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2018, pp. 183-269;
(b) Q.Z. Zheng, N. Jiao, Chem. Soc. Rev. 45 (2016) 4590-4627;
(c) M. Yan, J.C. Lo, J.T. Edwards, P.S. Baran, J. Am. Chem. Soc. 138 (2016) 12692-12714;
(d) Q. Feng, Y.W. Zhao, Q.L. Song, Chin. Chem. Lett. 27 (2016) 571-574;
(e) Y. Wei, P. Hu, M. Zhang, W. Su, Chem. Rev. 117 (2017) 8864-8907;
(f) G. Fang, X. Cong, G. Zanoni, Q. Liu, X. Bi, Adv. Synth. Catal. 359 (2017) 1422-1502;
(g) J. Schwarz, B. König, Green Chem. 20 (2018) 323-361;
(h) X. Yin, W. Li, B. Zhao, C. Kai, Chin. J. Org. Chem. 38 (2018) 2879-2887;
(i) R.S. J. Proctor, R.J. Phipps, Angew. Chem. Int. Ed. 58 (2019) 13666-13699;
(j) S. Wang, Z. Fu, Z. Huang, Y. Jiang, S. Guo, H. Cai, Chin. Chem. Lett. 30 (2019) 1173-1177.
-
[16]
Z. Wang, L. Zhu, F. Yin, et al., J. Am. Chem. Soc. 134(2012) 4258-4263.
doi: 10.1021/ja210361z
-
[17]
(a) F. Yin, Z. Wang, Z. Li, C. Li, J. Am. Chem. Soc. 134 (2012) 10401-10404;
(b) X. Liu, Z. Wang, X. Cheng, C. Li, J. Am. Chem. Soc. 134 (2012) 14330-14333;
(c) W.P. Mai, J.T. Wang, L.R. Yang, et al., Org. Lett. 16 (2014) 204-207;
(d) P.F. Wang, X.Q. Wang, J.J. Dai, Y.S. Feng, H.J. Xu, Org. Lett. 16 (2014) 4586-4589;
(e) F. Hu, X. Shao, D. Zhu, L. Lu, Q. Shen, Angew. Chem. Int. Ed. 53 (2014) 6105-6109;
(f) C. Liu, X. Wang, Z. Li, L. Cui, C. Li, J. Am. Chem. Soc. 137 (2015) 9820-9823;
(g) Y. Zhu, X. Li, X. Wang, et al., Org. Lett. 17 (2015) 4702-4705;
(h) P. Natarajan, R. Chaudhary, P.J. Venugopalan, J. Org. Chem. 80 (2015) 10498-10504;
(i) G. Shi, C. Shao, S. Pan, et al., Org. Lett. 17 (2015) 38-41;
(j) X.F. Xia, S.L. Zhu, C. Chen, H. Wang, Y.M. Liang, J. Org. Chem. 81 (2016) 1277-1284;
(k) D. Zhu, X. Shao, X. Hong, L. Lu, Q. Shen, Angew. Chem. Int. Ed. 55 (2016) 15807-15811;
(l) L. Cui, H. Chen, C. Liu, C. Li, Org. Lett. 18 (2016) 2188-2191;
(m) Q.W. Zhang, A.T. Brusoe, V. Mascitti, et al., Angew. Chem. Int. Ed. 55 (2016) 9758-9762;
(n) Y. Zhu, X. Wen, S. Song, N. Jiao, ACS Catal. 6 (2016) 6465-6472;
(o) L. Lv, S. Lu, Y. Chen, Z. Li, Org. Chem. Front. 4 (2017) 2147-2152;
(p) X. Tan, Z. Liu, H. Shen, et al., J. Am. Chem. Soc. 139 (2017) 12430-12433;
(q) X. Tan, T. Song, Z. Wang, et al., Org. Lett. 19 (2017) 1634-1637;
(r) Y. Dong, Z. Wang, C. Li, Nat. Commun. 8 (2017) 277;
(s) W. Shu, A. Lorente, E. Gómez-Bengoa, C. Nevado, Nat. Commun. 8 (2017)13832;
(t) R.G. Xing, Y.N. Li, B.W. Zhang, Chin. Chem. Lett. 28 (2017) 407-411;
(u) Y. Guo, M.W. Huang, X.L. Fu, et al., Chin. Chem. Lett. 28 (2017) 719-728;
(v) H. Hu, X. Chen, K. Su, et al., Org. Chem. Front. 5 (2018) 2925-2929;
(w) K. Sun, S.J. Li, X.L. Chen, et al., Chem. Commun. 55 (2019) 2861-2864;
(x) Z. Wang, C.Y. Guo, C. Yang, J.P. Chen, J. Am. Chem. Soc. 141 (2019) 5617-5622;
(y) C.G. Li, Q. Xie, X.L. Xu, et al., Org. Lett. 21 (2019) 8496-8500;
(z) R. Matsubara, H. Kim, T. Sakaguchi, et al., Org. Lett. 22 (2020) 1182-1187.
-
[18]
(a) R. Xia, M.S. Xie, H.Y. Niu, G.R. Qu, H.M. Guo, Org. Lett. 16 (2014) 444-447;
(b) W.M. Zhao, X.L. Chen, J.W. Yuan, et al., Chem. Commun. 50 (2014) 2018-2020;
(c) W.P. Mai, B. Sun, L.Q. You, et al., Org. Biomol. Chem. 13 (2015) 2750-2755;
(d) P.S. Mahajan, S.B. Mhaske, Org. Lett. 20 (2018) 2092-2095;
(e) J.Y. Guo, T. Guan, J.Y. Tao, K. Zhao, T.P. Loh, Org. Lett. 21 (2019) 8395-8399.
-
[19]
(a) Z. Yan, B. Wu, X. Gao, M.W. Chen, Y.G. Zhou, Org. Lett. 18 (2016) 692-695;
(b) Z.B. Zhao, L. Shi, Y. Li, F.J. Meng, Y.G. Zhou, Org. Biomol. Chem. 17 (2019) 6364-6368;
(c) Z.B. Zhao, L. Shi, F.J. Meng, Y. Li, Y.G. Zhou, Org. Chem. Front. 6 (2019) 1572-1576.

 Login In
Login In






 DownLoad:
DownLoad: