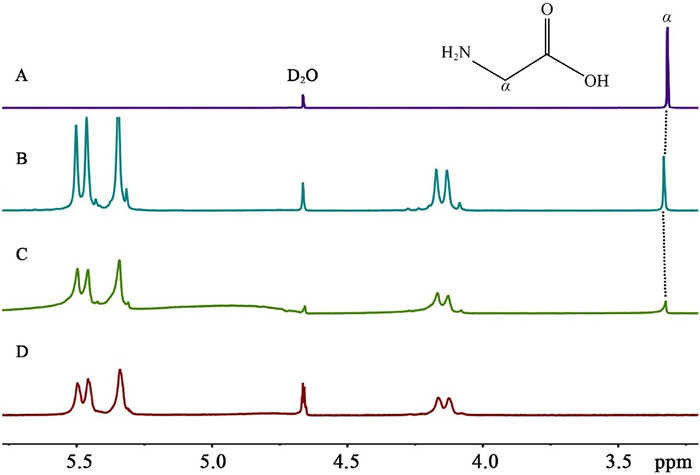
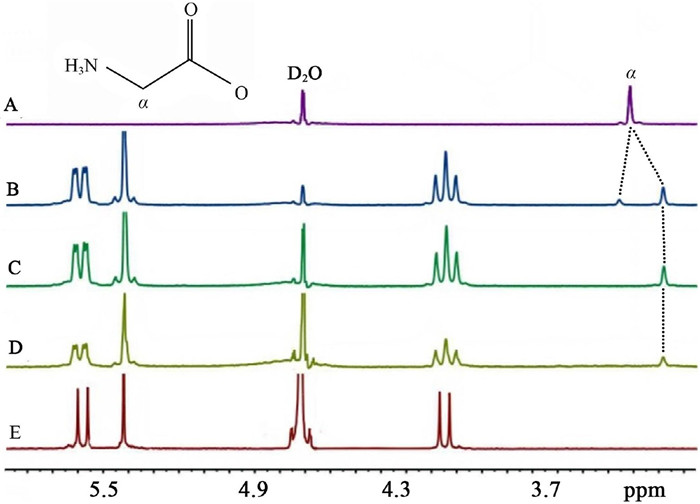
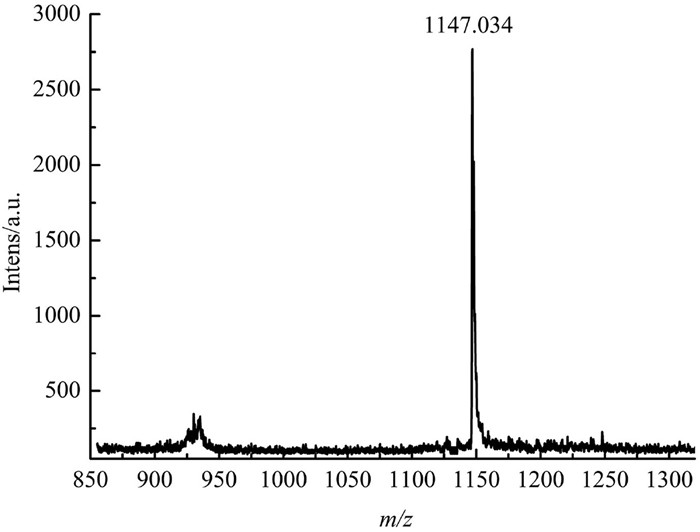
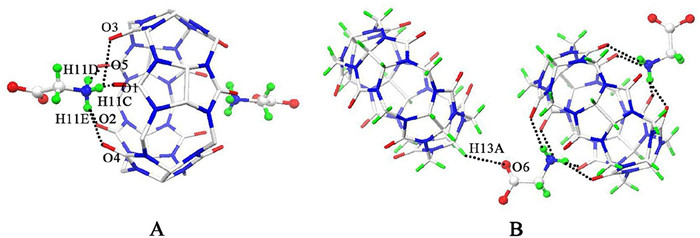
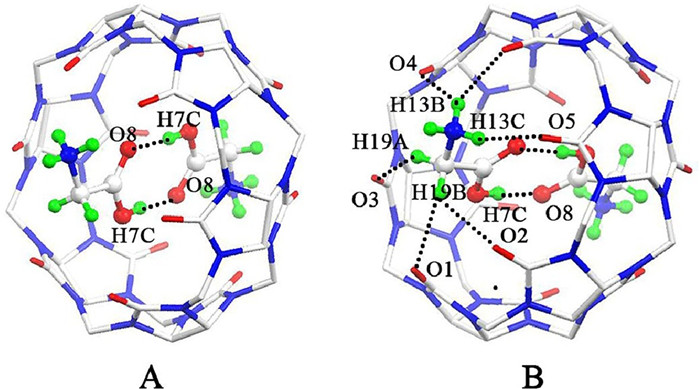
-
[1]
(a) M. Freidman, J. Agric. Food Chem. 47 (1999) 3457-3479;
(b) Y. Zhou, J. Yoon, Chem. Soc. Rev. 41 (2012) 52-67;
(c) H. Heli, M. Hajjizadeh, A. Jabbari, A. Moosavi-Movahedi, Anal. Biochem. 388 (2009) 81-90;
(d) Q.P. Duan, Y. Cao, Y. Li, et al., J. Am. Chem. Soc. 135 (2013) 10542-10549;
(e) B.B. Shi, K.C. Jie, Y.J. Zhou, et al., J. Am. Chem. Soc. 138 (2016) 80-83;
(f) G.C. Yu, G.P. Tang, F.H. Huang, J. Mater. Chem. C 2 (2014) 6609-6617;
(g) G.C. Yu, D. Wu, Y. Li, et al., Chem. Sci. 7 (2016) 3017-3024;
(h) Y. Cao, X.Y. Hu, Y. Li, et al., J. Am. Chem. Soc. 136 (2014) 10762-10769;
(i) M. Liu, J.L. Kan, Y.Q. Yao, et al., Sensors Actuators B: Chem. 283 (2019) 290-297;
(j) R.H. Gao, L.X. Chen, K. Chen, Z. Tao, X. Xiao, Coord. Chem. Rev. 348 (2017) 1–24.
-
[2]
(a) C.B. Lebrilla, Acc. Chem. Res. 34 (2001) 653-661;
(b) W. Si, P. Xin, Z.T. Li, J.L. Hou, Acc. Chem. Res. 48 (2015) 1612–1619;
(c) A. Casnati, F. Sansone, R. Ungaro, Acc. Chem. Res. 36 (2003) 246-254;
(d) K. Yang, Y. Pei, J. Wen, Z. Pei, Chem. Commun. 52 (2016) 9316–9326;
(e) M. Giuliani, I. Morbioli, F. Sansone, A. Casnati, Chem. Commun. 51 (2015)14140–14159;
(f) R. Pinalli, A. Pedrini, E. Dalcanale, Chem. Soc. Rev. 47 (2018) 7006-7026.
-
[3]
(a) A.R. Urbach, V. Isr. Ramalingam, J. Chem. 51 (2011) 664;
(b) A.T. Wright, M.J. Griffin, Z.L. Zhong, et al., Angew. Chem. Int. Ed. 44 (2005) 6375-6378;
(c) Q.H. Bai, S.W. Zhang, H.R. Chen, et al., ChemistrySelect 2 (2017) 2569-2573.
-
[4]
(a) T.B. Wei, J.F. Chen, X.B. Cheng, et al., Org. Chem. Front. 4 (2017) 210-213;
(b) L. Chen, W. Si, L. Zhang, et al., J. Am. Chem. Soc. 135 (2013) 2152-2155;
(c) S. Tashiro, M. Tominaga, M. Kawano, et al., J. Am. Chem. Soc. 127 (2005) 4546-4547;
(d) P. Wang, Z.T. Li, X.F. Ji, Chem. Commun. 50 (2014) 13114-13116;
(e) C.J. Li, J.W. Ma, L. Zhao, et al., Chem. Commun. 49 (2013) 1924-1926;
(f) B. Hua, J. Zhou, G.C. Yu, Tetrahedron Lett. 56 (2015) 986-989.
-
[5]
(a) J.P. Chinta, A. Acharya, A. Kumar, C.P. Rao, J. Phys. Chem. B 113 (2009) 12075-12083;
(b) R.K. Pathak, J. Dessingou, C.P. Rao, Anal. Chem. 84 (2012) 8294-8300;
(c) R.K. Pathak, V.K. Hinge, K. Mahesh, et al., Anal. Chem. 84 (2012) 6907-6913;
(d) J. Dessingou, A. Mitra, K. Tabbasum, G.S. Baghel, C.P. Rao, J. Org. Chem. 77 (2012) 371-378;
(e) R. Joseph, B. Ramanujam, A. Acharya, C.P. Rao, J. Org. Chem. 74 (2009) 8181-8190.
-
[6]
(a) J. Lagona, P. Mukhopadhyay, S. Chakrabarti, L. Isaacs, Angew. Chem. Int. Ed. 44 (2005) 4844-4870;
(b) X.L. Ni, X. Xiao, H. Cong, et al., Chem. Soc. Rev. 42 (2013) 9480-9508;
(c) E. Masson, X. Ling, R. Joseph, L. Kyeremeh-Mensah, X. Lu, RSC Adv. 2 (2012) 1213-1247;
(d) K.I. Assaf, W.M. Nau, Chem. Soc. Rev. 44 (2015) 394-418;
(e) S.J. Barrow, S. Kasera, M.J. Rowland, J. Barrio, O.A. Scherman, Chem. Rev. 115 (2015) 12320-12406;
(f) X.L. Ni, S.Y. Chen, Y.P. Yang, Z. Tao, J. Am. Chem. Soc. 138 (2016) 6177-6183.
-
[7]
(a) L.A. Logsdon, C.L. Schardon, V. Ramalingam, S.K. Kwee, A.R. Urbach, J. Am.
Chem. Soc. 133 (2011) 17087-17092;
(b) J.M. Chinai, A.B. Taylor, L.M. Ryno, et al., J. Am. Chem. Soc. 133 (2011) 8810-8813;
(c) L.A. Logsdon, A.R. Urbach, J. Am. Chem. Soc. 135 (2013) 11414-11416;
(d) L.C. Smith, D.G. Leach, B.E. Blaylock, O.A. Ali, A.R. Urbach, J. Am. Chem. Soc. 137 (2015) 3663-3669.
-
[8]
(a) M.V. Rekharsky, H. Yamamura, Y.H. Ko, et al., Chem. Commun. (2008) 2236-2238;
(b) W.J. Kim, K. Kim, H.I. Kim, Angew. Chem. Int. Ed. 126 (2014) 7591-7595;
(c) J.W. Lee, H.H.L. Lee, Y.H. Ko, K. Kim, H.I. Kim, J. Phys. Chem. B 119 (2015) 4628-4636;
(d) L.T. Li, M. Liu, L.D. Yue, et al., Anal. Chem. 92 (2020) 9322-9329;
(e) L.D. Yue, C. Sun, Q. Cheng, et al., Chem. Commun. 55 (2019) 13506-13509;
(f) S. Combes, K.T. Tran, M.M. Ayhan, et al., J. Am. Chem. Soc. 141 (2019) 5897-5907.
-
[9]
(a) A. Hennig, H. Bakirci, W.M. Nau, Nat. Methods 4 (2007) 629-632;
(b) D.M. Bailey, A. Hennig, V.D. Uzunova, W.M. Nau, Chem. Eur. J. 14 (2008) 6069-6077;
(c) A. Praetorius, D.M. Bailey, T. Schwarzlose, W.M. Nau, Org. Lett. 10 (2008) 4089-4092;
(d) W.M. Nau, G. Ghale, A. Hennig, H. Bakirci, D.M. Bailey, J. Am. Chem. Soc. 131 (2009) 11558-11570;
(e) H. Yin, X.J. Zhang, J.W. Wei, et al., Theranostics 11 (2021) 1513-1526;
(f) H. Yin, D. Bardelang, R.B. Wang, Trends Analyt. Chem. 3 (2021) 1-4.
-
[10]
M. Pozo, P. Hernàndez, L. Hernàndez, C.J. Quintana, Mater. Chem. 21 (2011) 13657–13663.
-
[11]
(a) S. Sonzini, T.J.S. Ryan, O.A. Scherman, Chem. Commun. 49 (2013) 8779-8781;
(b) K.I. Kuok, S.K. Li, Ian W. Wyman, R.B. Wang, Ann. N.Y. Acad. Sci. 1398 (2017) 108-119.
-
[12]
M.A. Gamal-Eldin, D.H. Macartney, Org. Biomol. Chem. 11 (2013) 488-495.
doi: 10.1039/C2OB27007B
-
[13]
T. Minami, N.A. Esipenko, B. Zhang, L. Isaacs, P. Anzenbacher Jr, Chem.
Commun. 50 (2014) 61–63.
doi: 10.1039/C3CC47416J
-
[14]
Y. He, Y. Liang, H. Yu, ACS Comb. Sci. 17 (2015) 409-412.
doi: 10.1021/acscombsci.5b00045
-
[15]
(a) D.T. Dang, H.D. Nguyen, M. Merkx, L. Brunsveld, Angew. Chem. Int. Ed. 52 (2013) 2915-2919;
(b) H.D. Nguyen, D.T. Dang, J.L.J. van Dongen, L. Brunsveld, Angew. Chem. Int. Ed. 49 (2010) 895-898.
-
[16]
O. Danylyuk, V.P. Fedin, Cryst. Growth Des. 12 (2012) 550-555.
doi: 10.1021/cg2013914
-
[17]
(a) P. Thuéry, Inorg. Chem. 50 (2011) 10558-10560;
(b) P. Thuéry, CrystEngComm 14 (2012) 8128-8136.
-
[18]
(a) J.M. Yi, Y.Q. Zhang, H. Cong, S.F. Xue, Z. Tao, J. Mol. Struct. 933 (2009) 112-117;
(b) P.H. Shan, S.C. Tu, R.L. Lin, et al., CrystEngComm 19 (2017) 2168-2171;
(c) T. Yin, S. Zhang, M.X. Li, C. Redshaw, X.L. Ni, Sens. Actuator. 281 (2019) 568-573.
-
[19]
(a) K. Moon, A.E. Kaifer, Org. Lett. 6 (2004) 185;
(b) A.E. Kaifer, Acc. Chem. Res. 47 (2014) 2160-2167;
(c) R.L. Lin, J.Q. Li, J.X. Liu, A.E. Kaifer, J. Org. Chem. 80 (2015) 10505-10511.
-
[20]
(a) Y.X. Qu, R.L. Lin, Y.Q. Zhang, et al., Org. Chem. Front. 4 (2017) 1799-1805;
(b) R.L. Lin, G.S. Fang, W.Q. Sun, J.X. Liu, Sci. Rep. 6 (2016);
(c) R.L. Lin, Y.P. Dong, Y.F. Hu, et al., RSC Adv. 2 (2012) 7754-7758.
-
[21]
(a) Y.H. Ko, E. Kim, I. Hwang, K. Kim, Chem. Commun. (2007) 1305-1315;
(b) Y. Liu, Y. Yu, J. Gao, Z. Wang, X. Zhang, Angew. Chem. Int. Ed. 49 (2010) 6576-6579;
(c) F. Biedermann, O.A. Scherman, J. Phys. Chem. B 116 (2012) 2842-2849;
(d) Z. Ji, J. Li, G. Chen, M. Jiang, ACS Macro Lett. 5 (2016) 588-592.
-
[22]
(a) F. Biedermann, V.D. Uzunova, O.A. Scherman, W.M. Nau, A.D. Simone, J. Am.
Chem. Soc. 134 (2012) 15318-15323;
(b) F. Biedermann, M. Vendruscolo, O.A. Scherman, A.D. Simone, W.M. Nau, J.
Am. Chem. Soc. 135 (2013) 14879-14888.

 Login In
Login In

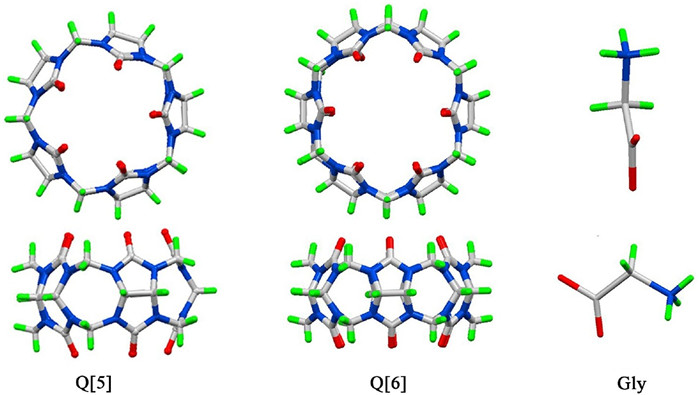





 DownLoad:
DownLoad: