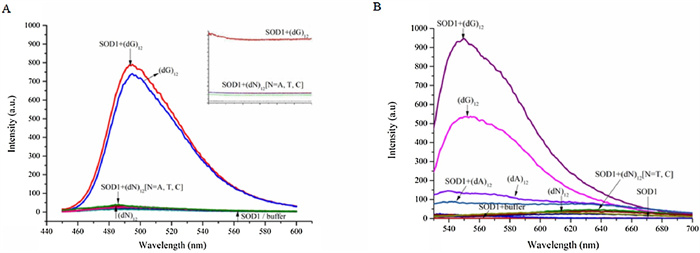
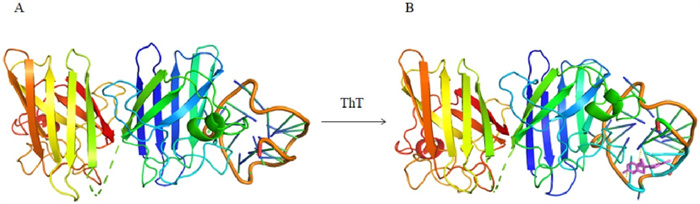
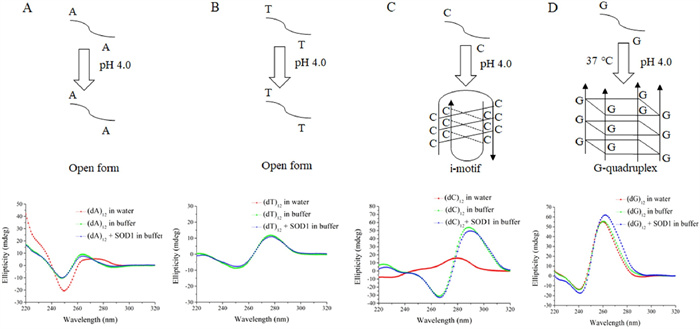
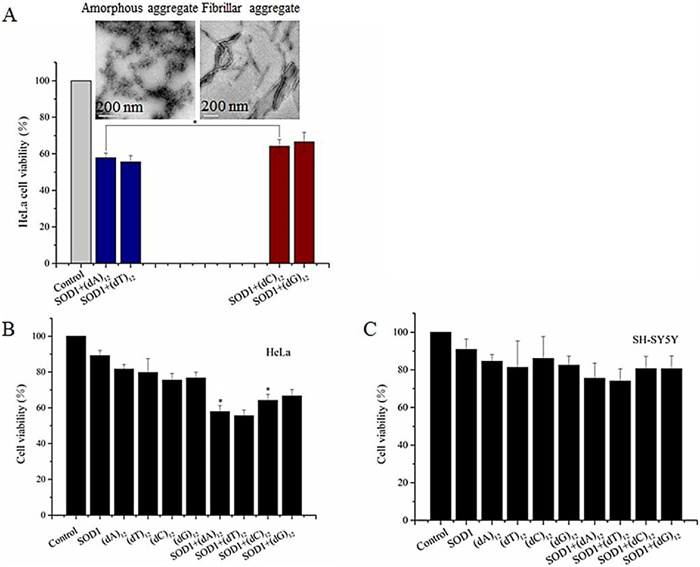
-
[1]
M. Calamai, J.R. Kumita, J. Mifsud, et al., Biochem. 45 (2006) 12806-12815.
doi: 10.1021/bi0610653
-
[2]
J.D. Domizio, R. Zhang, L.J. Stagg, et al., J. Biol. Chem. 287 (2012) 736-747.
doi: 10.1074/jbc.M111.238477
-
[3]
C.L. Liu, Y. Zhang, Adv. Protein Chem. Struct. Biol. 84 (2011) 1-40.
doi: 10.1145/2037715.2037759
-
[4]
J.D. Domizio, S. Dorta-Estremera, M. Gagea, et al., Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 14550 ̵ 14555.
doi: 10.1073/pnas.1206923109
-
[5]
C.N. Wong, L.W. Xiong, M. Horiuchi, et al., EMBO J. 20 (2001) 377-386.
doi: 10.1093/emboj/20.3.377
-
[6]
B. Macedo, T.A. Millen, C.A.C.A. Braga, et al., Biochem. 51 (2012) 5402-5413.
doi: 10.1021/bi300440e
-
[7]
S.F. Banani, A.M. Rice, W.B. Peeples, et al., Cell 166 (2016) 651-663.
doi: 10.1016/j.cell.2016.06.010
-
[8]
A. Khong, T. Matheny, S. Jain, et al., Cell 68 (2017) 808-820.
doi: 10.1016/j.molcel.2017.10.015
-
[9]
P. Ivanov, E. O'Day, M.M. Emara, et al., Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 18201-18206.
doi: 10.1073/pnas.1407361111
-
[10]
Y. Zhang, C. Roland, C. Sagui, Neurosci. 9 (2018) 1104-1117.
doi: 10.1021/acschemneuro.7b00476
-
[11]
X. Li, J.Y. Shi, S. Qiu, et al., Prog. Chem. 30 (2018) 1475-1486.
-
[12]
X. Li, Y. Chen, J. Zhao, et al., Oxid. Med. Cell. Longev. 2019 (2019) 9706792.
-
[13]
B. J. Carter, P. Anklesaria, S. Choi, et al., Antioxid. Redox Sign. 11 (2009) 1569-1586.
doi: 10.1089/ars.2008.2414
-
[14]
A. E. Frakes, L. Ferraiuolo, A. M. Haidet-Phillips, et al., Neuron 81 (2014) 1009-1023.
doi: 10.1016/j.neuron.2014.01.013
-
[15]
X. Li, S. Qiu, J. Shi, et al., Nucleic Acids Res. 47 (2019) 5074-5085.
doi: 10.1093/nar/gkz256
-
[16]
C.K. Tsang, M. Chen, X. Cheng, et al., Mol. Cell 70 (2018) 502-515.
doi: 10.1016/j.molcel.2018.03.029
-
[17]
A.R. Reddi, V.C. Culotta, Cell 152 (2013) 224-235.
doi: 10.1016/j.cell.2012.11.046
-
[18]
W. Jiang, Y.C. Han, R.Y. Zhou, et al., Biochem. 46 (2007) 5911-5923.
doi: 10.1021/bi062234m
-
[19]
W. Jiang, B. Zhang, J. Yin, et al., Biopolymers 89 (2008) 1154-1169.
doi: 10.1002/bip.21067
-
[20]
J. Yin, S. Hu, W. Jiang, et al., PLoS One 5 (2010) e12328.
doi: 10.1371/journal.pone.0012328
-
[21]
J. Yin, R.Z. Chen, C.L. Liu, Front. Biosci. 14 (2009) 5084-5106.
doi: 10.2741/3588
-
[22]
Y. Yue, F. Huo, P. Yue, et al., Anal. Chem. 90 (2018) 7018-7024.
doi: 10.1021/acs.analchem.8b01406
-
[23]
D. S. Leeman, K, Hebestreit, T. Ruetz, et al., Science 359 (2018) 1277-1283.
doi: 10.1126/science.aag3048
-
[24]
G. Habicht, C. Haupt, R.P. Friedrich, et al., Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 19232-19237.
doi: 10.1073/pnas.0703793104
-
[25]
G.C.P. van Zundert, J.P.G.L.M. Rodrigues, M. Trellet, et al., J. Mol. Biol. 428 (2015) 720-725.
doi: 10.1016/j.jmb.2015.09.014
-
[26]
D. Zhao, X. Dong, N. Jiang, et al., Nucleic Acids Res. 42 (2014) 11612-11622.
doi: 10.1093/nar/gku833
-
[27]
S. Stefansson, D.L. Adams, C.M. Tang, et al., BioTechniques 52 (2012) 1-6.
doi: 10.1155/2012/371487
-
[28]
J. Choi, S. Kim, T. Tachikawa, et al., J. Am. Chem. Soc. 133 (2011) 16146-16153.
doi: 10.1021/ja2061984
-
[29]
A. Rajendran, S.I. Nakano, N. Sugimoto, Chem. Commun. 46 (2010) 1299-1301
doi: 10.1039/b922050j
-
[30]
S. Pramanik, S. Nagatoishi, N. Sugimoto, Chem. Commun. 48 (2012) 4815-4817.
doi: 10.1039/c2cc30622k
-
[31]
R. Jin, B.L. Gaffney, C. Wang, et al., Proc. Natl. Acad. Sci. U. S. A. 89 (1992) 8832-8836.
doi: 10.1073/pnas.89.18.8832
-
[32]
A. Rajendran, B.U. Nair. BBA-Gen, Subjects 1760 (2006) 1794-1801.
doi: 10.1016/j.bbagen.2006.08.011
-
[33]
C.M. Karch, M. Prudencio, D.D. Winkler, et al., Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 7774-7779.
doi: 10.1073/pnas.0902505106
-
[34]
M. Chattopadhyay, A. Durazo, S.H. Sohn, et al., Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 18663-18668.
doi: 10.1073/pnas.0807058105
-
[35]
Z.A.O. Durer, J.A. Cohlberg, P. Dinh, et al., PLoS One 4 (2009) e5004.
doi: 10.1371/journal.pone.0005004
-
[36]
J.S. Elam, A.B. Taylor, R. Strange, et al., Nat. Struct. Mol. Biol. 10 (2003) 461-467.
doi: 10.1038/nsb935
-
[37]
M. Bucciantini, E. Giannoni, F. Chiti, et al., Nature 416 (2002) 507-511.
doi: 10.1038/416507a
-
[38]
I. Choi, Y.I. Yang, H.D. Song, et al., Biochim. Biophys. Acta 1812 (2011) 41-48.
doi: 10.1016/j.bbadis.2010.09.003
-
[39]
S.J. Weisberg, R. Lyakhovetsky, A.C. Werdiger, et al., Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 15811-15816.
doi: 10.1073/pnas.1205829109
-
[40]
G.W. Collie, R. Promontorio, S.M. Hampel, et al., J. Am. Chem. Soc. 134 (2012) 2723-2731.
doi: 10.1021/ja2102423

 Login In
Login In






 DownLoad:
DownLoad: