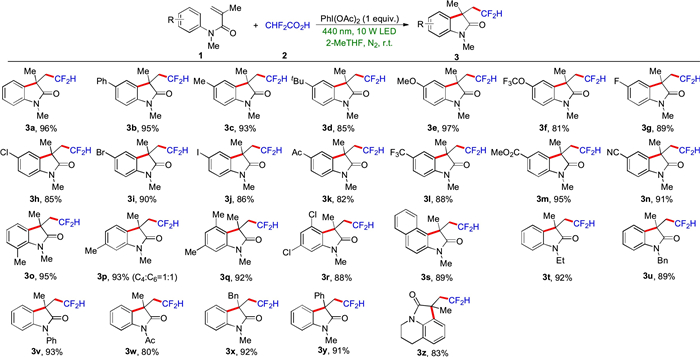
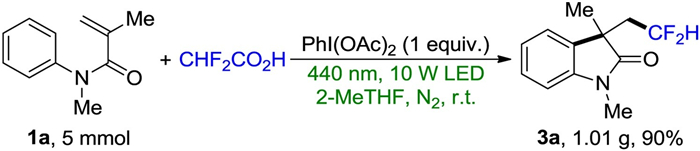
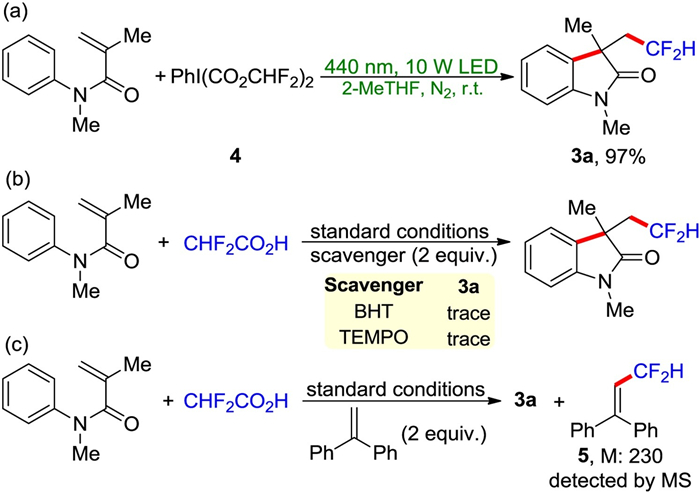
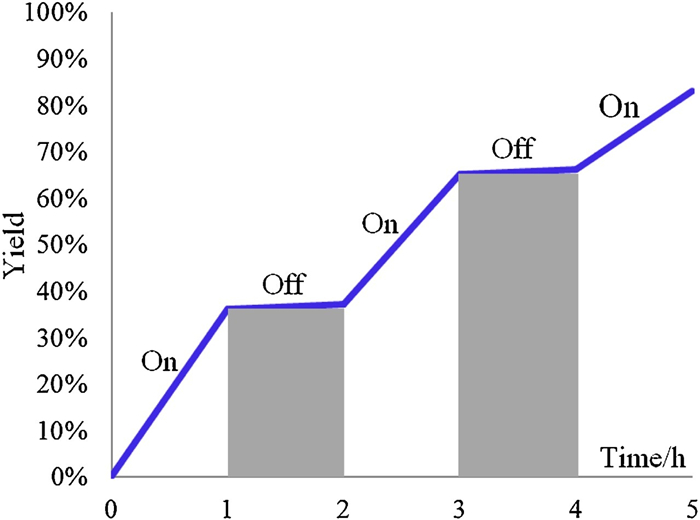
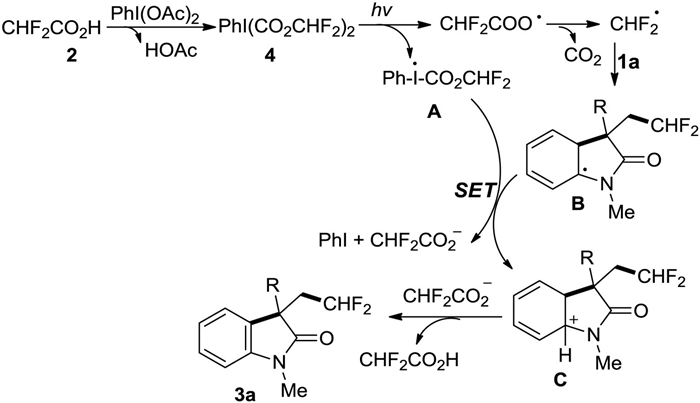
-
[1]
(a) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138;
(b) Y. Kim, C.J. Li, Green Synth. Catal. 1 (2020) 1-11.
-
[2]
(a) C.J. Clarke, W.C. Tu, O. Levers, A. Bröhl, J.P. Hallett, Chem. Rev. 118 (2018) 747-800;
(b) M. Li, F. Wu, Y. Gu, Chin. J. Catal. 40 (2019) 1135-1140;
(c) L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240;
(d) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290;
(e) Q. Sun, L. Liu, Y. Yang, Z. Zha, Z. Wang, Chin. Chem. Lett. 30 (2019) 1379-1382;
(f) F. Gao, R. Bai, F. Ferlin, et al., Green Chem. 22 (2020) 6240-6257;
(g) F.H. Qin, X.J. Huang, Y. Liu, et al., Chin. Chem. Lett. 31 (2020) 3267-3270.
-
[3]
(a) Q. Zhen, L. Chen, L. Qi, et al., Chem. Asian J. 15 (2020) 106-111;
(b) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30 (2019) 2259-2262;
(c) Y. Wang, J. Shen, Q. Chen, L. Wang, M. He, Chin. Chem. Lett. 30 (2019) 409-412.
-
[4]
(a) F. Wang, T.Q. Wei, P. Xu, S.Y. Wang, S.J. Ji, Chin. Chem. Lett. 30 (2019) 379-382;
(b) Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361-1368;
(c) J. Chen, L. Wu, J. Wu, Chin. Chem. Lett. 31 (2020) 2993-2995;
(d) F.S. He, Y. Yao, W. Xie, J. Wu, Chem. Commun. 56 (2020) 9469-9472;
(e) Q.W. Gui, F. Teng, S.N. Ying, et al., Chin. Chem. Lett. 31 (2020) 3241-3244.
-
[5]
(a) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30 (2019) 2295-2298;
(b) D.Q. Dong, L.X. Li, G.H. Li, et al., Chin. J. Catal. 40 (2019) 1494-1498;
(c) M. Fu, X. Ji, Y. Li, G.J. Deng, H. Huang, Green Chem. 22 (2020) 5594-5598;
(d) L. Lu, Y. Li, X. Jiang, Green Chem. 22 (2020) 5989-5994;
(e) F.S. He, S. Xie, Y. Yao, J. Wu, Chin. Chem. Lett. 31 (2020) 3065-3072;
(f) L. Zou, L. Wang, L. Sun, X. Xie, P. Li, Chem. Commun. 56 (2020) 7933-7936;
(g) S. He, X. Chen, F. Zeng, et al., Chin. Chem. Lett. 31 (2020) 1863-1867;
(h) W. Xiao, J. Wu, Chin. Chem. Lett. 31 (2020) 3083-3094;
(i) P. Wang, Q. Zhao, W. Xiao, J. Chen, Green Synth. Catal. 1 (2020) 42-51.
-
[6]
(a) Y. Liu, X.L. Chen, K. Sun, et al., Org. Lett. 21 (2019) 4019-4024;
(b) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1613;
(c) J. Shi, W. Wei, Chin. J. Org. Chem. 40 (2020) 2170-2172;
(d) Z. Gan, G. Li, X. Yang, et al., Sci. China Chem. 63 (2020) 1652;
(e) L.Y. Xie, Y.S. Bai, X.Q. Xu, et al., Green Chem. 22 (2020) 1720-1725;
(f) J. Xu, H. Zhang, J. Zhao, et al., Org. Chem. Front. 7 (2020) 4031-4042;
(g) W.B. He, L.Q. Gao, X.J. Chen, et al., Chin. Chem. Lett. 31 (2020) 1895-1898.
-
[7]
(a) J. Bergman, Oxindoles, in: E.F.V. Scriven, C.A. Ramsden (Eds.), Advances in Heterocyclic Chemistry, Vol. 117, Academic Press, 2015, pp. 1-81;
(b) N. Ye, H. Chen, E.A. Wold, P.Y. Shi, J. Zhou, ACS Infect. Dis. 2 (2016) 382-392;
(c) M.Z. Zhang, L. Liu, Q. Gou, et al., Green Chem. 22 (2020) 8369-8374;
(d) Y. An, Y. Li, J. Wu, Org. Chem. Front. 3 (2016) 570-573;
(e) C. Wang, Q. Chen, Q. Guo, et al., J. Org. Chem. 81 (2016) 5782-5788;
(f) K. Zhou, J. Chen, J. Wu, Chin. Chem. Lett. 31 (2020) 2996-2998.
-
[8]
(a) Z.Z. Han, C.P. Zhang, Adv. Synth. Catal. 362 (2020) 4256-4292;
(b) A. Lemos, C. Lemaire, A. Luxen, Adv. Synth. Catal. 361 (2019) 1500-1537;
(c) W. Sha, W. Zhang, S. Ni, et al., J. Org. Chem. 82 (2017) 9824-9831; (d) Y. Zhou, Z. Xiong, J. Qiu, L. Kong, G. Zhu, Org. Chem. Front. 6 (2019) 1022-1026;
(e) C.F. Meyer, S.M. Hell, A. Misale, A.A. Trabanco, V. Gouverneur, Angew. Chem. Int. Ed. 58 (2019) 8829-8833;
(f) R. Xu, C. Cai, Chem. Commun. 55 (2019) 4383-4386;
(g) D. Zhang, Z. Fang, J. Cai, et al., Chem. Commun. 56 (2020) 8119-8122;
(h) D.Q. Dong, H. Yang, J.L. Shi, et al., Org. Chem. Front. 7 (2020) 2538-2575.
-
[9]
(a) Y. Xiang, Y. Li, Y. Kuang, J. Wu, Chem. Eur. J. 23 (2017) 1032-1035;
(b) J.Y. Wang, Y.M. Su, F. Yin, et al., Chem. Commun. 50 (2014) 4108-4111;
(c) J.Y. Wang, X. Zhang, Y. Bao, et al., Org. Biomol. Chem. 12 (2014) 5582-5585.
-
[10]
J. Liu, S. Zhuang, Q. Gui, et al., Eur. J. Org. Chem. (2014) 3196-3202.
-
[11]
X.J. Tang, C.S. Thomoson, W.R. Dolbier, Org. Lett. 16(2014) 4594-4597.
doi: 10.1021/ol502163f
-
[12]
H. Sun, Y. Jiang, Y.S. Yang, et al., Org. Biomol. Chem. 17(2019) 6629-6638.
doi: 10.1039/C9OB01213C
-
[13]
P. Xiong, H.H. Xu, J. Song, H.C. Xu, J. Am. Chem. Soc. 140(2018) 2460-2464.
doi: 10.1021/jacs.8b00391
-
[14]
Z. Ruan, Z. Huang, Z. Xu, et al., Org. Lett. 21(2019) 1237-1240.
doi: 10.1021/acs.orglett.9b00361
-
[15]
(a) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. China Chem. 62 (2019) 460-464;
(b) W.M. He, Y.W. Lin, D.H. Yu, Sci. China Chem. 63 (2020) 291-293;
(c) L.Y. Xie, T.G. Fang, J.X. Tan, et al., Green Chem. 21 (2019) 3858-3863;
(d) K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30 (2019) 2304-2308;
(e) K.J. Liu, J.H. Deng, T.Y. Zeng, et al., Chin. Chem. Lett. 31 (2020) 1868-1872;
(f) Y. Wu, W. He, Chin. J. Org. Chem. 40 (2020) 2597-2599;
(g) L.Y. Xie, Y.S. Liu, H.R. Ding, et al., Chin. J. Catal. 41 (2020) 1168-1173;
(h) J.Y. Chen, C.T. Zhong, Q.W. Gui, et al., Chin. Chem. Lett. 32 (2021) 475-479;
(i) W.T. Wei, Q. Li, M.Z. Zhang, W.M. He, Chin. J. Catal. 42 (2021) 731-742.
-
[16]
R. Sakamoto, H. Kashiwagi, K. Maruoka, Org. Lett. 19(2017) 5126-5129.
doi: 10.1021/acs.orglett.7b02416
-
[17]
(a) Y. Mei, L. Zhao, Q. Liu, et al., Green Chem. 22 (2020) 2270-2278;
(b) D. Xia, Y. Li, T. Miao, P. Li, L. Wang, Green Chem. 19 (2017) 1732-1739.

 Login In
Login In

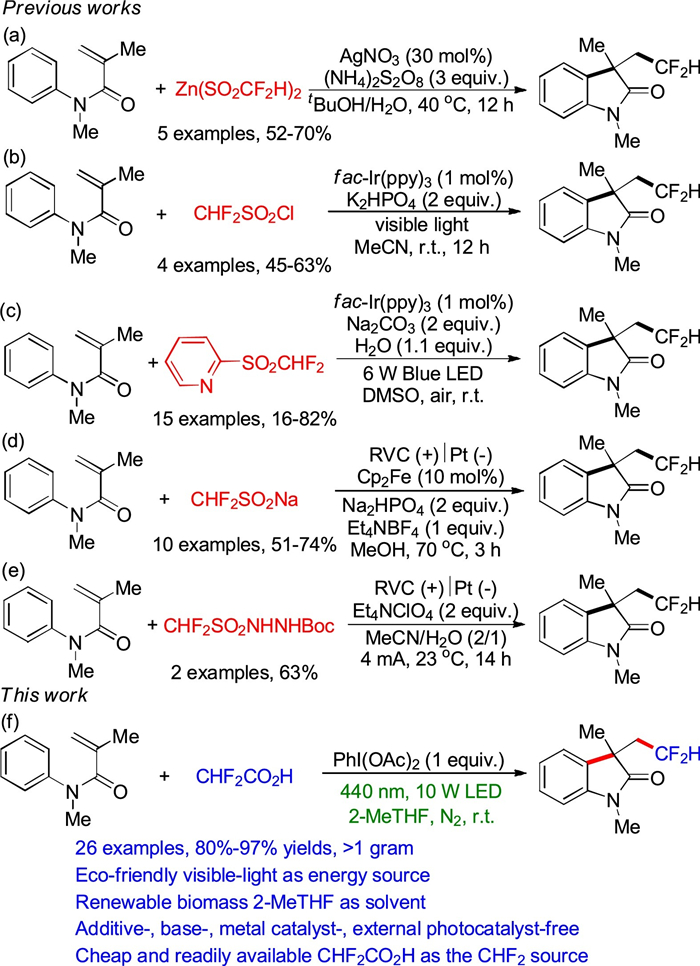





 DownLoad:
DownLoad: