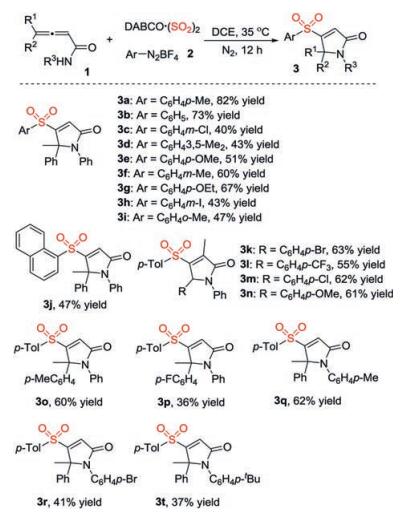
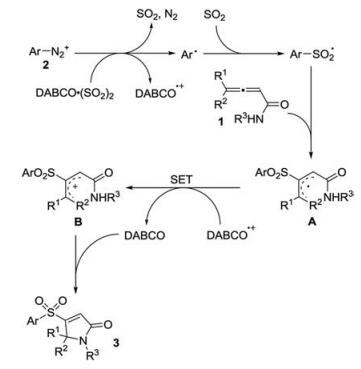
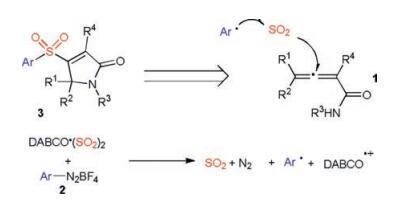
-
[1]
(a) K.C. Nicolaou, L. Shi, M. Lu, et al., Angew. Chem. Int. Ed. 53 (2014) 10970-10974;
(b) H. Uchiro, N. Shionozaki, R. Tanaka, et al., Tetrahedron Lett. 54 (2013) 506-511;
(c) R. Tanaka, K. Ohishi, N. Takanashi, et al., Org. Lett. 14 (2012) 4886-4889.
-
[2]
(a) L.N. Kirpotina, I.A. Schepetkin, A.I. Khlebnikov, et al., Biochem. Pharmacol. 142 (2017) 120-132;
(b) K. Ma, P. Wang, W. Fu, et al., Bioorg. Med. Chem. Lett. 21 (2011) 6724-6727;
(c) C. Zhuang, Z. Miao, L. Zhu, et al., J. Med. Chem. 55 (2012) 9630-9642;
(d) C. Peifer, R. Selig, K. Kinkel, et al., J. Med. Chem. 51 (2008) 3814-3824.
-
[3]
X. del Corte, A. López-Francés, A. Maestro, et al., J. Org. Chem. 85(2020) 14369-14383.
doi: 10.1021/acs.joc.0c00280
-
[4]
(a) K.E.O. Ylijoki, J.M. Stryker, Chem. Rev. 113 (2013) 2244-2266;
(b) M.E. Garst, B.J. McBride, J.G. Douglass Ⅲ, Tetrahedron Lett. 24 (1983) 1675-1678.
-
[5]
(a) J.T. Palmer, D. Rasnick, J.L. Klaus, D. Brömme, J. Med. Chem. 38 (1995) 3193-3196;
(b) D.C. Meadows, T.B. Mathews, T.W. North, et al., J. Med. Chem. 48 (2005) 4526-4534;
(c) D.C. Meadows, J. Gervay-Hague, Med. Res. Rev. 26(2006) 793-814;
(d) R. Ettari, E. Nizi, M.E. Di Francesco, et al., J. Med. Chem. 51 (2008) 988-996;
(e) R. van der Westhuyzen, E. Strauss, J. Am. Chem. Soc. 132 (2010) 12853-12855.
-
[6]
(a) G. Qiu, K. Zhou, L. Gao, J. Wu, Org. Chem. Front. 5 (2018) 691-705;
(b) K. Hofman, N. Liu, G. Manolikakes, Chem. Eur. J. 24 (2018) 11852-11863;
(c) G. Liu, C. Fan, J. Wu, Org. Biomol. Chem. 13 (2015) 1592-1599;
(d) G. Qiu, L. Lai, J. Cheng, J. Wu, Chem. Commun. 54 (2018) 10405-10414;
(e) G. Qiu, K. Zhou, J. Wu, Chem. Commun. 54 (2018) 12561-12569;
(f) A.S. Deeming, E.J. Emmett, C.S. Richards-Taylor, M.C. Willis, Synthesis 46 (2014) 2701-2710;
(g) S. Ye, M. Yang, J. Wu, Chem. Commun. 56 (2020) 4145-4155;
(h) S. Ye, G. Qiu, J. Wu, Chem. Commun. 55 (2019) 1013-1019;
(i) F.S. He, M. Yang, S. Ye, J. Wu, Chin. Chem. Lett. 31 (2020), doi: http://dx.doi.org/10.1016/j.cclet.2020.04.043.
-
[7]
(a) D. Zheng, Y. An, Z. Li, J. Wu, Angew. Chem. Int. Ed. 53 (2014) 2451-2454;
(b) D. Zheng, J. Yu, J. Wu, Angew. Chem. Int. Ed. 55 (2016) 11925-11929;
(c) X. Gong, M. Wang, S. Ye, J. Wu, Org. Lett. 21 (2019) 1156-1160;
(d) S. Ye, D. Zheng, J. Wu, G. Qiu, Chem. Commun. 55 (2019) 2214-2217;
(e) S. Ye, Y. Li, J. Wu, Z. Li, Chem. Commun. 55 (2019) 2489-2492;
(f) X. Gong, X. Li, W. Xie, J. Wu, S. Ye, Org. Chem. Front. 6 (2019) 1863-1867;
(g) S. Ye, T. Xiang, X. Li, J. Wu, Org. Chem. Front. 6 (2019) 2183-2199;
(h) J. Zhang, W. Xie, S. Ye, J. Wu, Org. Chem. Front. 6 (2019) 2254-2259;
(i) F.S. He, X. Gong, P. Rojsitthisak, J. Wu, J. Org. Chem. 84 (2019) 13159-13163;
(j) S. Ye, X. Li, W. Xie, J. Wu, Asian J. Org. Chem. 8 (2019) 893-898.
-
[8]
(a) K. Zhou, J.B. Liu, W. Xie, S. Ye, J. Wu, Chem. Commun. 56 (2020) 2554-2557;
(b) S. Ye, K. Zhou, P. Rojsitthisak, J. Wu, Org. Chem. Front. 7 (2020) 14-18;
(c) J. Zhang, M. Yang, J.B. Liu, F.S. He, J. Wu, Chem. Commun. 56 (2020) 3225-3228;
(d) X. Gong, M. Yang, J.B. Liu, F.S. He, X. Fan, J. Wu, Green Chem. 22 (2020) 1906-1910;
(e) X. Gong, M. Yang, J.B. Liu, F.S. He, J. Wu, Org. Chem. Front. 7 (2020) 938-943.
-
[9]
(a) Y. Li, S. Chen, M. Wang, X. Jiang, Angew. Chem. Int. Ed. 59 (2020) 8907-8911;
(b) Y. Meng, M. Wang, X. Jiang, Angew. Chem. Int. Ed. 59(2020) 1346-1353;
(c) C.M. Huang, J. Li, S.Y. Wang, S.J. Ji, Chin. Chem. Lett. 31 (2020) 1923-1926;
(d) M. Wang, J. Zhao, X. Jiang, ChemSusChem 12 (2019) 3064-3068;
(e) M. Wang, Q. Fan, X. Jiang, Green Chem. 20 (2018) 5469-5473;
(f) L.W. Ye, C. Shua, F. Gagosz, Org. Biomol. Chem. 12 (2014) 1833-1845;
(g) Y. Park, S. Chang, Nat. Catal. 2 (2019) 219-227;
(h) Q. Xing, C.M. Chan, Y.W. Yeung, W.Y. Yu, J. Am. Chem. Soc. 141 (2019) 3849-3853;
(i) B.H. Zhu, C.M. Wang, H.Y. Su, L.W. Ye, Chin. J. Chem. 37 (2019) 58-62;
(j) C. Shu, M.Q. Liu, S.S. Wang, L. Li, L.W. Ye, J. Org. Chem. 78 (2013) 3292-3299.

 Login In
Login In






 DownLoad:
DownLoad: