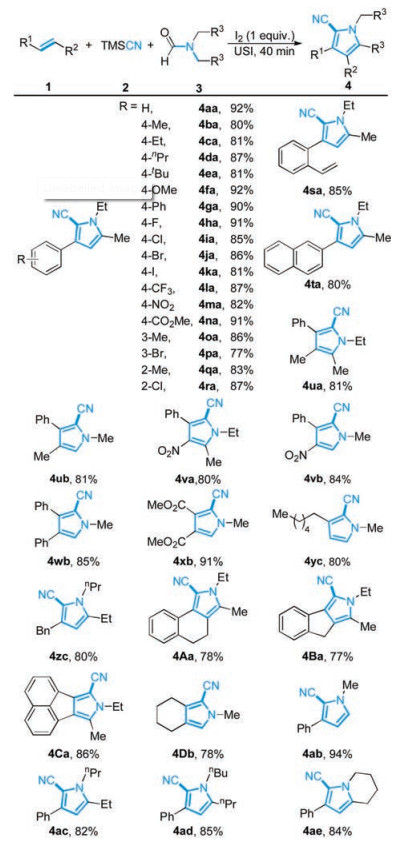
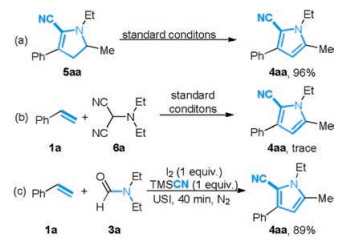
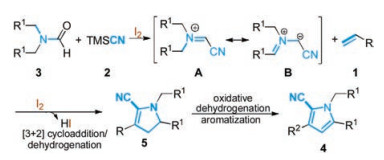
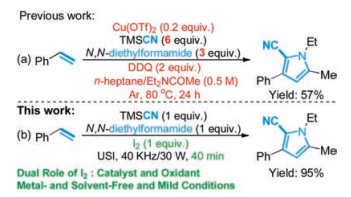
-
[1]
G. Chatel, R.S. Varma, Green Chem. 21(2019) 6043-6050.
-
[2]
(a) S.G. Pharande, A.R. Corrales Escobosa, R. Gamez-Montano, Green Chem.19(2017) 1259-1262;
(b) C. Wu, L.H. Lu, A.Z. Peng, et al., Green Chem. 20(2018) 3683-3688;
(c) L.H. Lu, S.J. Zhou, M. Sun, et al., ACS Sustain. Chem. Eng. 7(2019) 1574-1579.
-
[3]
(a) M.H. Huang, W.J. Hao, B. Jiang, Chem. Asian J. 13(2018) 2958-2977;
(b) M.H. Xu, K.L. Dai, Y.Q. Tu, et al., Chem. Commun. 54(2018) 7685-7688;
(c) S.S. Jiang, Y.C. Wu, S.Z. Luo, et al., Chem. Commun. 55(2019) 12805-12808;
(d) K. Sun, B. Luan, Z. Liu, et al., Org. Biomol. Chem. 17(2019) 4208-4211;
(e) C. Wan, R.J. Song, J.H. Li, Org. Lett. 21(2019) 2800-2803;
(f) H. Ruan, L.G. Meng, L. Zhu, L. Wang, Adv. Synth. Catal. 361(2019) 3217-3222;
(g) Q. Liu, F. Liu, H. Yue, et al., Adv. Synth. Catal. 361(2019) 5277-5282;
(h) L. Zhao, P. Li, H. Zhang, L. Wang, Org. Chem. Front. 6(2019) 87-93;
(i) G.P. Yang, X. Wu, B. Yu, C. Hu, ACS Sustain. Chem. Eng. 7(2019) 3727-3732;
(j) X. Wang, Y.F. Han, X.H. Ouyang, R.J. Song, J.H. Li, Chem. Commun. 55(2019) 14637-14640.
-
[4]
(a) S. Chen, Y. Li, P. Ni, H. Huang, G.J. Deng, Org. Lett. 18(2016) 5384-5387;
(b) H. Hu, X. Chen, K. Sun, et al., Org. Chem. Front. 5(2018) 2925-2929;
(c) F.L. Zeng, K. Sun, X.L. Chen, et al., Adv. Synth. Catal. 361(2019) 5176-5181;
(d) J. Lin, R.J. Song, M. Hu, J.H. Li, Chem. Rec. 19(2019) 440-451;
(e) X.C. Liu, K. Sun, Q.Y. Lv, et al., New J. Chem. 43(2019) 12221-12224;
(f) L. Zou, P. Li, B. Wang, L. Wang, Green Chem. 21(2019) 3362-3369.
-
[5]
(a) L. Wang, D. Xiong, L. Jie, C. Yu, X. Cui, Chin. Chem. Lett. 29(2018) 907-910;
(b) X. Gong, G. Li, Z. Gan, et al., Asian J. Org. Chem. 8(2019) 1472-1478;
(c) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30(2019) 2287-2290;
(d) G. Li, Q. Yan, X. Gong, X. Dou, D. Yang, ACS Sustain. Chem. Eng. 7(2019) 14009-14015;
(e) Z. Wang, W.M. He, Chin. J. Org. Chem. 39(2019) 3594-3595;
(f) L. Wang, Y. Zhang, M. Zhang, et al., Tetrahedron Lett. 60(2019) 1845-1848;
(g) W. Huang, J. Xu, C. Liu, Z. Chen, Y. Gu, J. Org. Chem. 84(2019) 2941-2950;
(h) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31(2020) 67-70;
(i)M. Li, X.Dong, N. Zhang, F. Jérôme, Y.Gu, GreenChem. 21(2019) 4650-4655;
(j) Y. Wu, C. Pi, X. Cui, Y. Wu, Org. Lett. 22(2020) 361-364.
-
[6]
(a) Z. Zhang, W. Zhang, J. Li, et al., J. Org. Chem. 79(2014) 11226-11233;
(b) A.H. Zhou, Q. He, C. Shu, et al., Chem. Sci. 6(2015) 1265-1271;
(c) C. Shu, Y.H. Wang, C.H. Shen, et al., Org. Lett. 18(2016) 3254-3257;
(d) Z.W. Gilbert, R.J. Hue, I.A. Tonks, Nat. Chem. 8(2016) 63-68;
(e) T. Li, H. Yan, X. Li, C. Wang, B. Wan, J. Org. Chem. 81(2016) 12031-12037;
(f) B.Y. Cheng, Y.N. Wang, T.R. Li, L.Q. Lu, W.J. Xiao, J. Org. Chem. 82(2017) 12134-12140;
(g) Y. Liu, X. Yi, X. Luo, C. Xi, J. Org. Chem. 82(2017) 11391-11398;
(h) A. Kumar, N. Tadigoppula Ramanand, Green Chem. 19(2017) 5385-5389;
(i) Z. Tian, J. Xu, B. Liu, Q. Tan, B. Xu, Org. Lett. 20(2018) 2603-2606;
(j) A. Kondoh, A. Iino, S. Ishikawa, T. Aoki, M. Terada, Chem. Eur. J. 24(2018) 15246-15253;
(k) L. Li, Q. Chen, X. Xiong, et al., Chin. Chem. Lett. 29(2018) 1893-1896;
(l) X.Q. Zhu, H. Yuan, Q. Sun, et al., Green Chem. 20(2018) 4287-4291;
(m) G. Cheng, W. Lv, L. Xue, Green Chem. 20(2018) 4414-4417;
(n) D. Chen, Y. Shan, J. Li, et al., Org. Lett. 21(2019) 4044-4048;
(o) J. Cen, Y. Wu, J. Li, et al., Org. Lett. 21(2019) 2090-2094;
(p) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. China Chem. 62(2019) 460-464;
(q) W. Huang, S. Chen, Z. Chen, et al., J. Org. Chem. 84(2019) 5655-5666;
(r) T. Schitter, S. Stammwitz, P.G. Jones, D.B. Werz, Org. Lett. 21(2019) 9415-9419;
(s) Q.W. Gui, X. He, W. Wang, et al., Green Chem. 22(2020) 118-122;
(t) G. Vengatesh, M. Sundaravadivelu, S. Muthusubramanian, J. Mol. Struct. 1199(2020) 126980;
(u) L. Qi, R. Li, X. Yao, et al., J. Org. Chem. 85(2020) 1097-1108.
-
[7]
(a) J. Jiang, H. Huang, G.J. Deng, Green Chem. 21(2019) 986-990;
(b) S. Liu, K. Chen, W.J. Hao, et al., J. Org. Chem. 84(2019) 1964-1971;
(c)Z.Xu, G.J.Deng, F.Zhang, H.Chen, H.Huang, Org.Lett.21(2019)8630-8634;
(d) H. Huang, Z. Qu, X. Ji, G.J. Deng, Org. Chem. Front. 6(2019) 1146-1150;
(e) X.Y. Qin, L. He, J. Li, et al., Chem. Commun. 55(2019) 3227-3230;
(f) S. Ye, X. Li, W. Xie, J. Wu, Asian J. Org. Chem. 8(2019) 893-898;
(g) G.H. Li, D.Q. Dong, Q. Deng, S.Q. Yan, Z.L. Wang, Synthesis 51(2019) 3313-3319;
(h) J. Xu, W. Huang, R. Bai, et al., Green Chem. 21(2019) 2061-2069.
-
[8]
X.Q. Mou, Z.L. Xu, L. Xu, et al., Org. Lett. 18(2016) 4032-4035.
doi: 10.1021/acs.orglett.6b01883
-
[9]
(a) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30(2019) 2259-2262;
(b) K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30(2019) 2304-2308;
(c) L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30(2019) 1237-1240;
(d) W.M. He, Y.W. Lin, D.H. Yu, Sci. China Chem. 63(2020) 291-293;
(e) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30(2019) 2132-2138.
-
[10]
(a) Z. Shi, L. Wang, X. Cui, Chin. J. Org. Chem. 39(2017) 1596-1612;
(b) H. Li, P. Zhou, F. Xie, et al., J. Org. Chem. 83(2018) 13335-13343;
(c) S.H. Hao, L.X. Li, D.Q. Dong, Z.L. Wang, Chin. J. Catal. 38(2017) 1664-1667;
(d) J. Ren, X. Yan, X. Cui, et al., Green Chem. 22(2020) 265-269;
(e) S. Du, C. Pi, T. Wan, Y. Wu, X. Cui, Adv. Synth. Catal. 361(2019) 1766-1770;
(f) P. Bao, L. Wang, H. Yue, et al., J. Org. Chem. 84(2019) 2976-2983.
-
[11]
(a) J.Y. Chen, Y.W. Lin, W.M. He, Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2020.03.034;
(b) W.B. He, L.Q. Gao, X.J. Chen, et al., Chin. Chem. Lett. 31(2020) 1895-1898;
(c) S. Peng, Y.W. Lin, W.M. He, Chin. J. Org. Chem. 40(2020) 541-542;
(d) F.H. Qin, X.J. Huang, Y. Liu, et al., Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2020.04.042;
(e) J.Y. Chen, H.Y. Wu, Q.W. Gui, et al., Org. Lett. 22(2020) 2206-2209.
-
[12]
X.Q. Mou, L. Xu, S.H. Wang, C. Yang, Tetrahedron Lett. 56(2015) 2820-2822.
doi: 10.1016/j.tetlet.2015.04.056

 Login In
Login In






 DownLoad:
DownLoad: