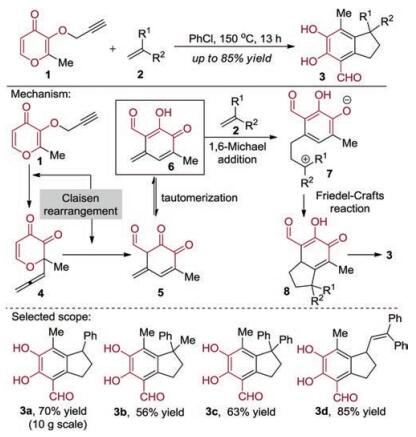
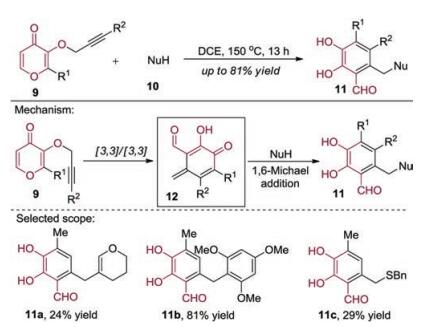
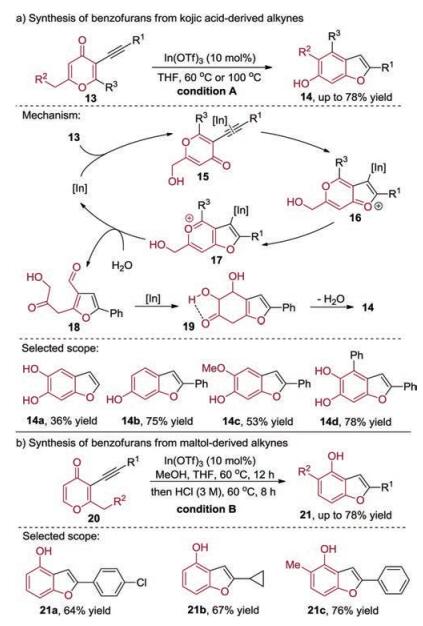
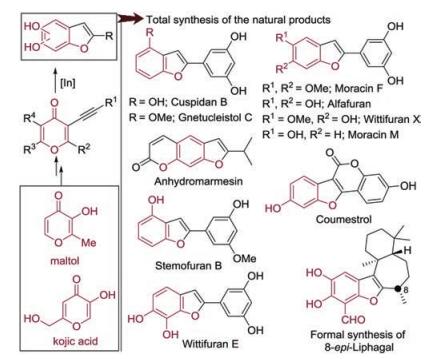
-
[1]
Wenling Yuan
, Fengli Li
, Zhe Chen
, Qiaoxin Xu
, Zhenhua Guan
, Nanyu Yao
, Zhengxi Hu
, Junjun Liu
, Yuan Zhou
, Ying Ye
, Yonghui Zhang
. AbnI: An α-ketoglutarate-dependent dioxygenase involved in brassicicene CH functionalization and ring system rearrangement. Chinese Chemical Letters,
2024, 35(5): 108788-.
doi: 10.1016/j.cclet.2023.108788
-
[2]
Peng Guo
, Shicheng Dong
, Xiang-Gui Zhang
, Bing-Bin Yang
, Jun Zhu
, Ke-Yin Ye
. Cobalt-catalyzed migratory carbon-carbon cross-coupling of borabicyclo[3.3.1]nonane (9-BBN) borates. Chinese Chemical Letters,
2025, 36(4): 110052-.
doi: 10.1016/j.cclet.2024.110052
-
[3]
Yuting Zhang
, Zhiqian Wang
. Methods and Case Studies for In-Depth Learning of the Aldol Reaction Based on Its Reversible Nature. University Chemistry,
2024, 39(7): 377-380.
doi: 10.3866/PKU.DXHX202311037
-
[4]
Nianhua Luo
, Jiayi Jiang
, Muhammad Suleman
, Zhaowen Liu
, Shuping Huang
, Wei Xiao
, Jie Wu
, Jiapian Huang
. Advances in radical Smiles rearrangement. Chinese Chemical Letters,
2026, 37(2): 111556-.
doi: 10.1016/j.cclet.2025.111556
-
[5]
Ying-Di Hao
, Zhi-Qian Lin
, Xiao-Yu Guo
, Jiao Liang
, Can-Kun Luo
, Qian-Tao Wang
, Li Guo
, Yong Wu
. Rhodium-catalyzed Doyle-Kirmse rearrangement reactions of sulfoxoniun ylides. Chinese Chemical Letters,
2024, 35(4): 108834-.
doi: 10.1016/j.cclet.2023.108834
-
[6]
Ji-Jia Zhou
, Li-Gao Liu
, Zhen-Tao Zhang
, Hao-Xuan Dong
, Xin Lu
, Zhou Xu
, Xin-Qi Zhu
, Bo Zhou
, Long-Wu Ye
. Copper-catalyzed asymmetric cascade diyne cyclization/Meinwald rearrangement. Chinese Chemical Letters,
2025, 36(9): 110870-.
doi: 10.1016/j.cclet.2025.110870
-
[7]
Wenyu Gao
, Liming Zhang
, Chuang Zhao
, Lixiang Liu
, Xingran Yang
, Jinbo Zhao
. Controlled semi-Pinacol rearrangement on a strained ring: Efficient access to multi-substituted cyclopropanes by group migration strategy. Chinese Chemical Letters,
2024, 35(9): 109447-.
doi: 10.1016/j.cclet.2023.109447
-
[8]
Ruru Li
, Qian Liu
, Hui Li
, Fengbin Sun
, Zhurui Shen
. Rational design of dual sites induced local electron rearrangement for enhanced photocatalytic oxygen activation. Chinese Chemical Letters,
2024, 35(11): 109679-.
doi: 10.1016/j.cclet.2024.109679
-
[9]
Huashan Huang
, Jingze Chen
, Luyun Zhang
, Hong Yan
, Siqi Li
, Fen-Er Chen
. Oscillatory flow reactor facilitates fast photochemical Wolff rearrangement toward synthesis of α-substituted amides in flow. Chinese Chemical Letters,
2025, 36(2): 109992-.
doi: 10.1016/j.cclet.2024.109992
-
[10]
Zhen Zhang
, Xue-ling Chen
, Xiu-Mei Xie
, Tian-Yu Gao
, Jing Qin
, Jun-Jie Li
, Chao Feng
, Da-Gang Yu
. Iron-promoted carbonylation–rearrangement of α-aminoaryl-tethered alkylidenecyclopropanes with CO2: Facile synthesis of quinolinofurans. Chinese Chemical Letters,
2025, 36(4): 110056-.
doi: 10.1016/j.cclet.2024.110056
-
[11]
Chonglong He
, Yulong Wang
, Quan-Xin Li
, Zichen Yan
, Keyuan Zhang
, Shao-Fei Ni
, Xin-Hua Duan
, Le Liu
. Alkylarylation of alkenes with arylsulfonylacetate as bifunctional reagent via photoredox radical addition/Smiles rearrangement cascade. Chinese Chemical Letters,
2025, 36(5): 110253-.
doi: 10.1016/j.cclet.2024.110253
-
[12]
Zhaoquan Guo
, Wenyao Ding
, Zhenguo Xi
, Lin Yang
, Gang Lu
, Hongyin Gao
. Protecting-group-dependent chemo- and regioselective cascade rearrangement of N-arylhydroxylamines with N-thiophthalimides. Chinese Chemical Letters,
2026, 37(2): 111196-.
doi: 10.1016/j.cclet.2025.111196
-
[13]
Wen-Rui Li
, Ru-Bing Wang
, Huiqiang Wang
, Jin-Yao Yong
, Yu-Huan Li
, Shi-Shan Yu
, Shuang-Gang Ma
. Ring-reorganization strategy for asymmetric synthesis of sesquiterpenoid illihenin A and its antiviral activity evaluation. Chinese Chemical Letters,
2025, 36(11): 110945-.
doi: 10.1016/j.cclet.2025.110945
-
[14]
Chenxi Shang
, Boxuan Lu
, Chongbei Wu
, Shuqing Zhou
, Luyan Shi
, Tayirjan Taylor Isimjan
, Xiulin Yang
. Inducing electronic rearrangement through Co3B-Mo2B5 catalysts: Efficient dual-function catalysis for NaBH4 hydrolysis and 4-nitrophenol reduction. Chinese Chemical Letters,
2025, 36(9): 111152-.
doi: 10.1016/j.cclet.2025.111152
-
[15]
Chuan-Zhi Ni
, Ruo-Ming Li
, Fang-Qi Zhang
, Qu-Ao-Wei Li
, Yuan-Yuan Zhu
, Jie Zeng
, Shuang-Xi Gu
. A chiral fluorescent probe for molecular recognition of basic amino acids in solutions and cells. Chinese Chemical Letters,
2024, 35(10): 109862-.
doi: 10.1016/j.cclet.2024.109862
-
[16]
Yanhui Zhong
, Peisi Xie
, Chengyi Xie
, Lei Guo
, Weiwei Chen
, Shuyi Wang
, Xiaoxiao Wang
, Fuyue Wang
, Zian Lin
, Gongke Li
, Zongwei Cai
. Rigid urea-based structures drive analysis of chiral amino acids. Chinese Chemical Letters,
2026, 37(2): 112039-.
doi: 10.1016/j.cclet.2025.112039
-
[17]
Zhen Dai
, Linzhi Tan
, Yeyu Su
, Kerui Zhao
, Yushun Tian
, Yu Liu
, Tao Liu
. Site-specific incorporation of reduction-controlled guest amino acids into proteins for cucurbituril recognition. Chinese Chemical Letters,
2024, 35(5): 109121-.
doi: 10.1016/j.cclet.2023.109121
-
[18]
Hongmei Yu
, Baoxi Zhang
, Meiju Liu
, Cheng Xing
, Guorong He
, Li Zhang
, Ningbo Gong
, Yang Lu
, Guanhua Du
. Theoretical and experimental cocrystal screening of temozolomide with a series of phenolic acids, promising cocrystal coformers. Chinese Chemical Letters,
2024, 35(5): 109032-.
doi: 10.1016/j.cclet.2023.109032
-
[19]
Liangji Chen
, Zhen Yuan
, Fudong Feng
, Xin Zhou
, Zhile Xiong
, Wuji Wei
, Hao Zhang
, Banglin Chen
, Shengchang Xiang
, Zhangjing Zhang
. A hydrogen-bonded organic framework containing fluorescent carbazole and responsive pyridyl units for sensing organic acids. Chinese Chemical Letters,
2024, 35(9): 109344-.
doi: 10.1016/j.cclet.2023.109344
-
[20]
Yanjing Li
, Jiayin Li
, Yuqi Chang
, Yunfeng Lin
, Lei Sui
. Tetrahedral framework nucleic acids promote the proliferation and differentiation potential of diabetic bone marrow mesenchymal stem cell. Chinese Chemical Letters,
2024, 35(9): 109414-.
doi: 10.1016/j.cclet.2023.109414

 Login In
Login In






 DownLoad:
DownLoad: