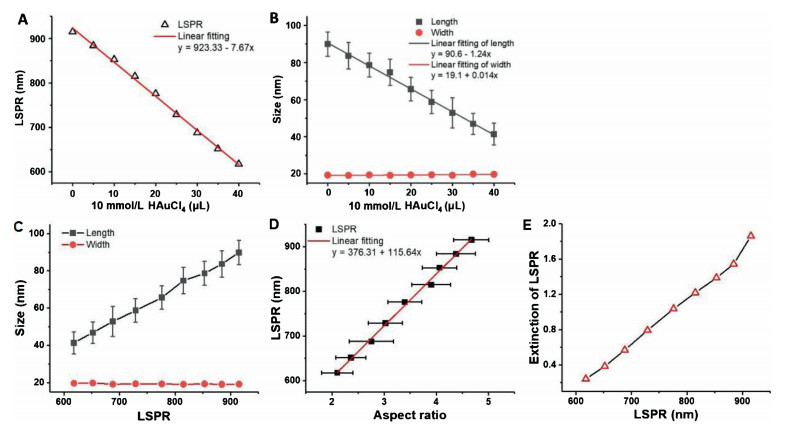
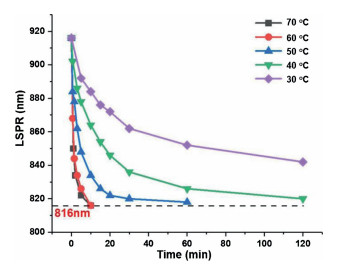
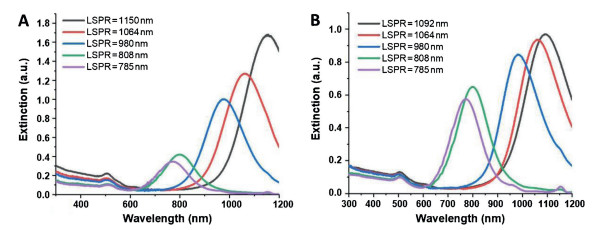
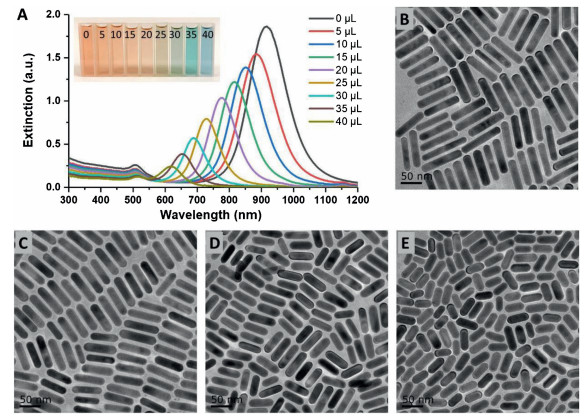
-
[1]
Min Huang
, Ru Cheng
, Shuai Wen
, Liangtong Li
, Jie Gao
, Xiaohui Zhao
, Chunmei Li
, Hongyan Zou
, Jian Wang
. Ultrasensitive detection of microRNA-21 in human serum based on the confinement effect enhanced chemical etching of gold nanorods. Chinese Chemical Letters,
2024, 35(9): 109379-.
doi: 10.1016/j.cclet.2023.109379
-
[2]
Chenkai Yang
, Xiaoling Pan
, Weiguang Zhao
, Zhiwen Qiu
, Lei He
, Cong Wu
, Ang Li
, Zhengnan Huang
, Yilin Yan
, Shengzhou Li
, Zhuofan Nan
, Xiangqian Cao
, Bing Shen
, Wei Li
. Intratumoral photo-controlled antigens burst release for synergistic immunotherapy by bio-membrane and organic membrane coated dual-functional nanoparticles. Chinese Chemical Letters,
2025, 36(9): 110740-.
doi: 10.1016/j.cclet.2024.110740
-
[3]
Hongpeng He
, Mengmeng Zhang
, Mengjiao Hao
, Wei Du
, Haibing Xia
. Synthesis of Different Aspect-Ratios of Fixed Width Gold Nanorods. Acta Physico-Chimica Sinica,
2024, 40(5): 2304043-0.
doi: 10.3866/PKU.WHXB202304043
-
[4]
Feng Shi
, Guiling Li
, Haibing Zhu
, Ling Li
, Ming Chen
, Juan Li
, Huifang Shen
, Hao Zeng
, Lingfeng Min
, Zhanjun Yang
. Nanozyme-triggered polymerization amplification strategy for constructing highly sensitive surface plasmon resonance immunosensing. Chinese Chemical Letters,
2025, 36(6): 110333-.
doi: 10.1016/j.cclet.2024.110333
-
[5]
Gang Hu
, Chun Wang
, Qinqin Wang
, Mingyuan Zhu
, Lihua Kang
. The controlled oxidation states of the H4PMo11VO40 catalyst induced by plasma for the selective oxidation of methacrolein. Chinese Chemical Letters,
2025, 36(2): 110298-.
doi: 10.1016/j.cclet.2024.110298
-
[6]
Xiaoqiang Wang
, Fangyuan Zhou
, Yue Liu
, Zhongbiao Wu
. CePO4 supported Cr catalyst with superior sulfur tolerance for selective catalytic oxidation of ammonia. Chinese Chemical Letters,
2025, 36(7): 110420-.
doi: 10.1016/j.cclet.2024.110420
-
[7]
Bo Li
, Yuanzhe Cheng
, Xuyang Ma
, Dongxu Zhao
, Yang Zhang
, Yongxing Sun
, Jia Chen
, Li Wu
, Liang Zhao
, Hongdeng Qiu
, Yujian He
. Facile and scale-up synthesis of cyano-functionalized covalent organic frameworks for selective gold recovery. Chinese Chemical Letters,
2026, 37(1): 111134-.
doi: 10.1016/j.cclet.2025.111134
-
[8]
Hao Zhang
, Haonan Qu
, Ehsan Bahojb Noruzi
, Haibing Li
, Feng Liang
. A nanocomposite film with layer-by-layer self-assembled gold nanospheres driven by cucurbit[7]uril for the selective transport of L-tryptophan and lysozyme. Chinese Chemical Letters,
2025, 36(1): 109731-.
doi: 10.1016/j.cclet.2024.109731
-
[9]
Zhijuan Niu
, Peizhe Sun
, Kwangnak Koh
, Changping Li
. Ultrasensitive electrochemical sensor based on para-sulfonatocalix[4]arene functionalized gold nanoparticles for sulfamethazine detection. Chinese Chemical Letters,
2025, 36(11): 110844-.
doi: 10.1016/j.cclet.2025.110844
-
[10]
Qiuyue Lu
, Min Shan
, Jiaqi Yang
, Zhongren Xu
, Yueyue Lei
, Wukun Liu
. A dinuclear gold(I) complex with bis(N-heterocyclic carbene) ligands potentiated immune responses against liver cancer via ROS-driven endoplasmic reticulum stress and ferroptosis. Chinese Chemical Letters,
2025, 36(12): 110940-.
doi: 10.1016/j.cclet.2025.110940
-
[11]
Zhipeng Wan
, Hao Xu
, Peng Wu
. Selective oxidation using in-situ generated hydrogen peroxide over titanosilicates. Chinese Journal of Structural Chemistry,
2024, 43(6): 100298-100298.
doi: 10.1016/j.cjsc.2024.100298
-
[12]
Shaonan Tian
, Yu Zhang
, Qing Zeng
, Junyu Zhong
, Hui Liu
, Lin Xu
, Jun Yang
. Core-shell gold-copper nanoparticles: Evolution of copper shells on gold cores at different gold/copper precursor ratios. Chinese Journal of Structural Chemistry,
2023, 42(11): 100160-100160.
doi: 10.1016/j.cjsc.2023.100160
-
[13]
Rong-Nan Yi
, Zi-Jian Zhao
, Wei-Min He
. Photoinduced gold-catalyzed cross-couplings. Chinese Chemical Letters,
2025, 36(7): 111070-.
doi: 10.1016/j.cclet.2025.111070
-
[14]
Dongmei Yao
, Junsheng Zheng
, Liming Jin
, Xiaomin Meng
, Zize Zhan
, Runlin Fan
, Cong Feng
, Pingwen Ming
. Effect of surface oxidation on the interfacial and mechanical properties in graphite/epoxy composites composite bipolar plates. Chinese Chemical Letters,
2024, 35(11): 109382-.
doi: 10.1016/j.cclet.2023.109382
-
[15]
Jia Chen
, Yun Liu
, Zerong Long
, Yan Li
, Hongdeng Qiu
. Colorimetric detection of α-glucosidase activity using Ni-CeO2 nanorods and its application to potential natural inhibitor screening. Chinese Chemical Letters,
2024, 35(9): 109463-.
doi: 10.1016/j.cclet.2023.109463
-
[16]
Jian Peng
, Yue Jiang
, Shuangyu Wu
, Yanran Cheng
, Jingyu Liang
, Yixin Wang
, Zhuo Li
, Sijie Lin
. A nonradical oxidation process initiated by Ti-peroxo complex showed high specificity toward the degradation of tetracycline antibiotics. Chinese Chemical Letters,
2024, 35(5): 108903-.
doi: 10.1016/j.cclet.2023.108903
-
[17]
Zhi Li
, Wenpei Li
, Shaoping Jiang
, Chuan Hu
, Yuanyu Huang
, Maxim Shevtsov
, Huile Gao
, Shaobo Ruan
. Legumain-triggered aggregable gold nanoparticles for enhanced intratumoral retention. Chinese Chemical Letters,
2024, 35(7): 109150-.
doi: 10.1016/j.cclet.2023.109150
-
[18]
Shimei Wu
, Yining Li
, Lantao Chen
, Yufei Zhang
, Lingxing Zeng
, Haosen Fan
. Hexapod cobalt phosphosulfide nanorods encapsulating into multiple hetero-atom doped carbon frameworks for advanced sodium/potassium ion battery anodes. Chinese Chemical Letters,
2025, 36(4): 109796-.
doi: 10.1016/j.cclet.2024.109796
-
[19]
Haoying ZHAI
, Lanzong WEN
, Wenjie LIAO
, Qin LI
, Wenjun ZHOU
, Kun CAO
. Metal-organic framework-derived sulfur-doped iron-cobalt tannate nanorods for efficient oxygen evolution reaction performance. Chinese Journal of Inorganic Chemistry,
2025, 41(5): 1037-1048.
doi: 10.11862/CJIC.20240320
-
[20]
Yunqing Zhu
, Kaiyue Wen
, Xuequan Wan
, Gaigai Dong
, Junfeng Niu
. High efficiency conversion of low concentration nitrate boosted with amorphous Cu0 nanorods prepared via in-situ reconstruction. Chinese Chemical Letters,
2025, 36(6): 110399-.
doi: 10.1016/j.cclet.2024.110399

 Login In
Login In






 DownLoad:
DownLoad: