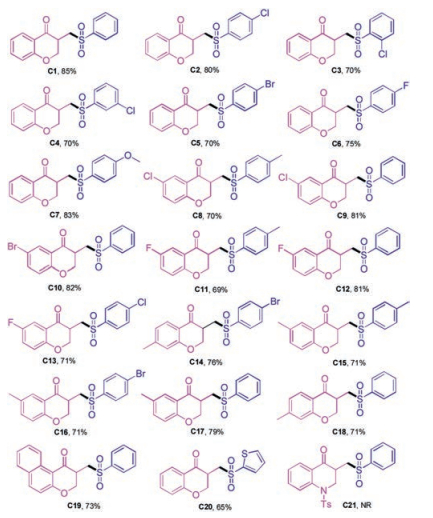
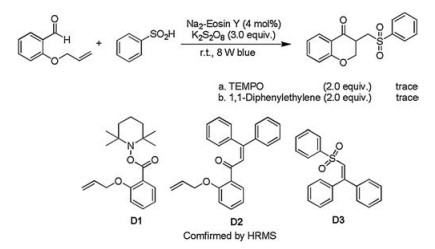
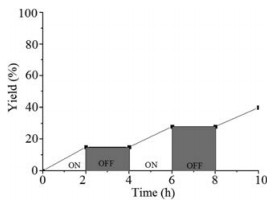
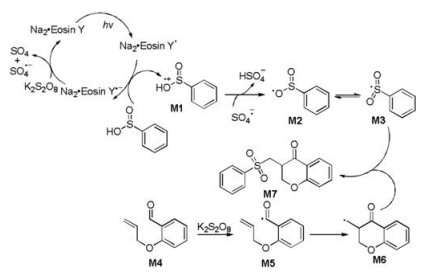
-
[1]
T. Seifert, M. Malo, T. Kokkola, et al., J. Med. Chem. 57(2014) 9870-9888.
doi: 10.1021/jm500930h
-
[2]
T. Nishida, H. Yoshinaga, T. Toyoda, M. Toshima, Org. Process Res. Dev.16(2012) 625-634.
doi: 10.1021/op200357h
-
[3]
A. Gaspar, M.J. Matos, J. Garrido, E. Uriarte, F. Borges, Chem. Rev. 114(2014) 4960-4992.
doi: 10.1021/cr400265z
-
[4]
J. Fan, W. Sun, Z. Wang, et al., Chem. Commun. 50(2014) 9573-9576.
doi: 10.1039/C4CC03778B
-
[5]
L. Peng, Z. Hu, Z. Tang, Y. Jiao, X. Xu, Chin. Chem. Lett. 30(2019) 1481-1487.
doi: 10.1016/j.cclet.2019.04.008
-
[6]
S. Jung, J. Kim, S. Hong, Adv. Synth. Catal. 359(2017) 3945-3949.
doi: 10.1002/adsc.201701072
-
[7]
N.N. Zhou, M.X. Wu, M. Zhang, X.Q. Zhou, Asian J. Org. Chem. 8(2019) 828-831.
doi: 10.1002/ajoc.201900121
-
[8]
M.Y. Chang, Y.S. Wu, H.Y. Chen, Org. Lett. 20(2018) 1824-1827.
doi: 10.1021/acs.orglett.8b00316
-
[9]
X.K. He, B.G. Cai, Q.Q. Yang, L. Wang, J. Xuan, Chem. Asian J. 14(2019) 3269-3273.
doi: 10.1002/asia.201901078
-
[10]
Q. Liu, G.Q. Xie, Q. Wang, et al., Tetrahedron 75(2019) 130490-130498.
doi: 10.1016/j.tet.2019.130490
-
[11]
J. Sheng, J.D. Liu, L.Q. Chen, et al., Org. Chem. Front. 6(2019) 1471-1495.
doi: 10.1039/C9QO00292H
-
[12]
(a) Y.M. Xiao, Y. Liu, W.P. Mai, et al., ChemistrySelect 4(2019) 1939-1942;
(b) D. Lu, Y. Wan, L. Kong, G. Zhu, Org. Lett. 19(2017) 2929-2932.
-
[13]
F.H. Xiao, C. Liu, D.H. Wang, H.W. Huang, G.J. Deng, GreenChem. 20(2018) 973-977.
-
[14]
W.C. Yang, P. Dai, K. Luo, Y.G. Ji, L. Wu, Adv. Synth. Catal. 359(2017) 2390-2395.
doi: 10.1002/adsc.201601407
-
[15]
J.J. Zhao, P. Li, X.J. Li, C.G. Xia, F.W. Li, Chem. Commun. 52(2016) 3661-3664.
doi: 10.1039/C5CC09730D
-
[16]
H. Hu, X.L. Chen, K. Sun, et al., Org. Lett. 20(2018) 6157-6160.
doi: 10.1021/acs.orglett.8b02627
-
[17]
H. Hu, X.L. Chen, K. Sun, et al., Org. Chem. Front. 5(2018) 2925-2929.
doi: 10.1039/C8QO00882E
-
[18]
L. Tang, Z. Yang, X.P. Chang, et al., Org. Lett. 20(2018) 6520-6525.
doi: 10.1021/acs.orglett.8b02846
-
[19]
J. Zhu, W.C. Yang, X.D. Wang, L. Wu, Adv. Synth. Catal. 360(2018) 386-400.
doi: 10.1002/adsc.201701194
-
[20]
(a) W.J. Zhou, Y.H. Zhang, Y.Y. Gui, L. Sun, D.G. Yu, Synthesis 50(2018) 3359-3378;
(b) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30(2019) 2132-2138.
-
[21]
K. Luo, W.C. Yang, L. Wu, Asian J. Org. Chem. 6(2017) 350-367.
doi: 10.1002/ajoc.201600512
-
[22]
C. Huang, X.B. Li, C.H. Tung, L.Z. Wu, Chem. Eur. J. 24(2018) 11530-11534.
doi: 10.1002/chem.201800391
-
[23]
(a) D. Staveness, I.C. Bosque, R.J. Stephenson, Acc. Chem. Res. 49(2016) 2295-2306;
(b) W. Yang, B. Li, M. Zhang, et al., Chin. Chem. Lett. 31(2020) 1313-1316;
(c) L.Y. Xie, Y.S. Lu, H.R. Ding, et al., Chin. J. Catal. 41(2020) 1168-1173.
-
[24]
(a) T. Courant, G. Masson, J. Org. Chem. 81(2016) 6945-6952;
(b) H. Xu, Y. Zhang, X. Lang, Chin. Chem. Lett. 31(2020) 1520-1524.
-
[25]
D.C. Fabry, M. Rueping, Acc. Chem. Res. 49(2016) 1969-1979.
doi: 10.1021/acs.accounts.6b00275
-
[26]
S. Cao, S.S. Zhong, L.T. Xin, J.P. Wan, C.P. Wen, ChemCatChem 7(2015) 1478-1482.
doi: 10.1002/cctc.201500139
-
[27]
S.Q. Zhu, A. Das, L. Bui, et al., J. Am. Chem. Soc. 135(2013) 1823-1829.
doi: 10.1021/ja309580a
-
[28]
X.Y. Yu, J.R. Chen, P.Z. Wang, et al., Angew. Chem. Int. Ed. 57(2018) 738-743.
doi: 10.1002/anie.201710618
-
[29]
H. Im, D. Kang, S. Choi, S. Shin, S. Hong, Org. Lett. 20(2018) 7437-7441.
doi: 10.1021/acs.orglett.8b03166
-
[30]
L. Zhang, J. Zhu, J. Ma, L. Wu, W.H. Zhang, Org. Lett. 19(2017) 6308-6311.
doi: 10.1021/acs.orglett.7b03052
-
[31]
G. Magallanes, M.D. Kärkäs, I. Bosque, et al., ACS Catal. 9(2019) 2252-2260.
doi: 10.1021/acscatal.8b04172
-
[32]
(a) H. Kaur, M. Kaur, P.K. Walia, M. Kumar, V. Bhalla, Chem. Asian J. 14(2019) 809-813;
(b) L.Y. Xie, J.L. Hu, Y.X. Song, et al., ACS Sustainable Chem. Eng. 7(2019) 19993-19999.
-
[33]
W. Wei, P.L. Bao, H.L. Yue, et al., Org. Lett. 20(2018) 5291-5295.
doi: 10.1021/acs.orglett.8b02231
-
[34]
M. Lanzi, J. Merad, D.V. Boyarskaya, et al., Org. Lett. 20(2018) 5247-5250.
doi: 10.1021/acs.orglett.8b02196
-
[35]
Q.M. Kainz, C.D. Matier, A. Bartoszewicz, et al., Science 351(2016) 681-684.
doi: 10.1126/science.aad8313
-
[36]
(a) T.Y. Shang, L.H. Lu, Z. Cao, et al., Chem. Commun. 55(2019) 5408-5419;
(b) W.B. He, L.Q. Gao, X.J. Chen, et al., Chin. Chem. Lett. 31(2020) 1895-1898.
-
[37]
Y. Gao, Y.Y. Liu, J.P. Wan, J. Org. Chem. 84(2019) 2243-2251.
doi: 10.1021/acs.joc.8b02981
-
[38]
(a) D. Yang, G. Li, C. Xing, et al., Org. Chem. Front. 5(2018) 2974-2979;
(b) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30(2019) 2295-2298.
-
[39]
Q.S. Liu, L.L. Wang, H.L. Yue, et al., Green Chem. 21(2019) 1609-1613.
doi: 10.1039/C9GC00222G
-
[40]
Y. Yasu, T. Koike, M. Akita, Org. Lett. 15(2013) 2136-2139.
doi: 10.1021/ol4006272
-
[41]
(a) G. Li, Q. Yan, Z. Gan, et al., Org. Lett. 21(2019) 7938-7940;
(b) X. Gong, Y. Ding, X. Fan, J. Wu, Adv. Synth. Catal. 359(2017) 2999-3004.
-
[42]
(a) J. Zhu, W.C. Yang, X.D. Wang, L. Wu, Adv. Synth. Catal. 360(2018) 386-400;
(b) W. Yang, S. Yang, P. Li, L. Wang, Chem. Commun. 51(2015) 7520-7523.
-
[43]
(a) X.S. Wu, Y. Chen, M.B. Li, M.G. Zhou, S.K. Tian, J. Am. Chem. Soc. 134(2012) 14694-14697;
(b) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31(2020) 67-70;
(c) L.Y. Xie, Y.L. Chen, L. Qin, et al., Org. Chem. Front. 6(2019) 3950-3955.
-
[44]
Y.M. Xi, B.L. Dong, E.J. McClain, et al., Angew. Chem. Int. Ed. 53(2014) 4657-4661.
doi: 10.1002/anie.201310142
-
[45]
(a) W. Wei, H. Cui, D. Yang, et al., Org. Chem. Front. 4(2017) 26-30;
(b) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31(2020) 67-70.
-
[46]
L.Y. Xie, S. Peng, F. Liu, et al., Org. Chem. Front. 5(2018) 2604-2609.
doi: 10.1039/C8QO00661J
-
[47]
W. Li, G. Yin, L. Huang, et al., Green Chem. 18(2016) 4879-4883.
doi: 10.1039/C6GC01196A
-
[48]
L. Xie, T. Fang, J. Tan, et al., Green Chem. 21(2019) 3858-3863.
doi: 10.1039/C9GC01175G
-
[49]
S. Kamijo, M. Hirota, K. Tao, M. Watanabe, T. Murafuji, Tetrahedron Lett. 55(2014) 5551-5554.
doi: 10.1016/j.tetlet.2014.08.011
-
[50]
(a) L.Y. Xie, S. Peng, J.X. Tan, et al., ACS Sustainable Chem. Eng. 6(2018) 16976-16981;
(b) P. Qian, Y. Deng, H. Mei, et al., Org. Lett. 19(2017) 4798-4801;
(c) L.Y. Xie, Y.J. Li, J. Qu, et al., Green Chem. 19(2017) 5642-5646;
(d) P. Bao, L. Wang, Q. Liu, et al., Tetrahedron Lett. 60(2019) 214-218.
-
[51]
S. Cai, Y. Xu, D. Chen, et al., Org. Lett. 18(2016) 2990-2993.
doi: 10.1021/acs.orglett.6b01353
-
[52]
(a) L. Wang, Y. Zhang, M. Zhang, et al., Tetrahedron Lett. 60(2019) 1845-1848;
(b) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30(2019) 2259-2262.
-
[53]
Y. Li, D. Zheng, Z. Li, J. Wu, Org. Chem. Front. 3(2016) 574-578.
doi: 10.1039/C6QO00060F
-
[54]
Y. Xiang, Y. Li, Y. Kuang, J. Wu, Chem. Eur. J. 23(2017) 1032-1035.
doi: 10.1002/chem.201605336
-
[55]
D.Q. Dong, L.X. Li, G.H. Li, et al., Chin. J. Catal. 40(2019) 1494-1498.
doi: 10.1016/S1872-2067(19)63420-0
-
[56]
G.H. Li, D.Q. Dong, Q. Deng, S.Q. Yan, Z.L. Wang, Synthesis 51(2019) 3313-3319.
doi: 10.1055/s-0037-1611787
-
[57]
(a) D.Q. Dong, W.J. Chen, Y. Yang, X. Gao, Z.L. Wang, ChemistrySelect 4(2019) 2480-2483;
(b) Q.Q. Han, G.H. Li, Y.Y. Sun, et al., Tetrahedron Lett. 61(2020) 151704.
-
[58]
(a) G.H. Li, D.Q. Dong, X.Y. Yu, Z.L. Wang, New J. Chem. 43(2019) 1667-1670;
(b) S.H. Hao, L.X. Li, D.Q. Dong, Z.L. Wang, Chin. J. Catal. 38(2017) 1664-1667;
(c) D.Q. Dong, S.H. Hao, H. Zhang, Z.L. Wang, Chin. Chem. Lett. 28(2017) 1597-1599.
-
[59]
(a) D.Q. Dong, W.J. Chen, D.M. Chen, et al., Chin. J. Org. Chem. 39(2019) 3190-3198;
(b) S. Yan, D.Q. Dong, C. Xie, W. Wang, Z.L. Wang, Chin. J. Org. Chem. 39(2019) 2560-2566.
-
[60]
G.H. Li, D.Q. Dong, Y. Yang, X.Y. Yu, Z.L. Wang, Adv. Synth. Catal. 361(2019) 832-835.
-
[61]
L.Y. Xie, T.G. Fang, J.X. Tan, et al., Green Chem. 21(2019) 3858-3863.
doi: 10.1039/C9GC01175G
-
[62]
(a) W. Wei, H. Cui, D. Yang, et al., Green Chem. 19(2017) 5608-5613;
(b) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30(2019) 2287-2290.

 Login In
Login In






 DownLoad:
DownLoad: