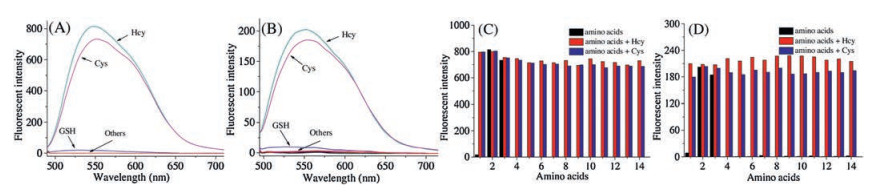
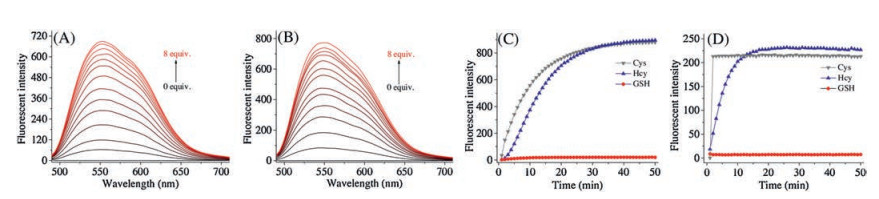
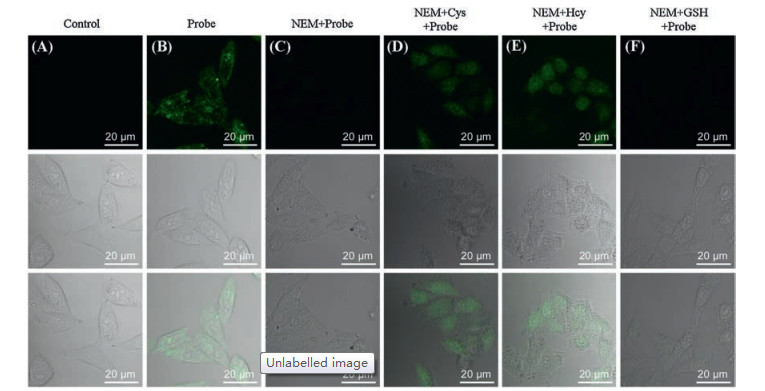
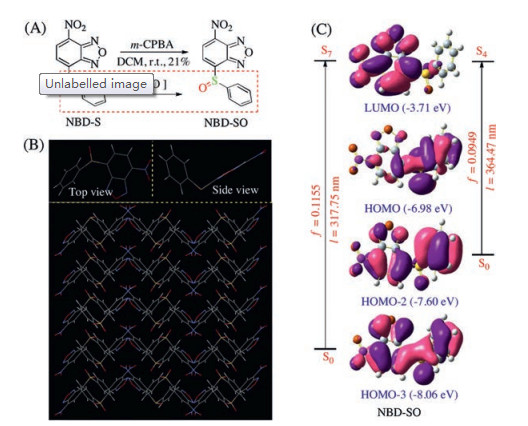
-
[1]
(a) S.Y. Zhang, C.N. Ong, H.M. Shen, Cancer Lett. 208 (2004) 143-153;
(b)Z.A.Wood, E.Schröder, J.R.Harris, L.B.Poole, TrendsBiochem.Sci.28 (2003)32-40.
-
[2]
(a) S. Shahrokhian, Anal. Chem. 73(2001) 5972-5978;
(b) T.P. Dalton, H.G. Shertzer, A. Puga, Annu. Rev. Pharmacol. Toxicol. 39 (1999) 67-101.
-
[3]
(a) Y.K. Yue, F.J. Huo, F.Q. Cheng, et al., Chem. Soc. Rev. 48 (2019) 4155-4177;
(b) Z.Q. Guo, Y.G. Ma, Y.J. Liu, et al., Sci. China Chem. 61 (2018) 1293-1300;
(c) L.J. Tang, M.G. Tian, H.B. Chen, et al., Dyes Pigm. 158 (2018) 482-489;
(d) P. Ning, W.J. Wang, M. Chen, Y. Feng, X.M. Meng, Chin. Chem. Lett. 28 (2017) 1943-1951.
-
[4]
(a) H. Zhang, K. Li, L.L. Li, et al., Chin. Chem. Lett. 30 (2019) 1063-1066;
(b) Y.K. Yue, F.J. Huo, X.Q. Li, et al., Org. Lett. 19 (2017) 82-85;
(c) F.J. Huo, Y.Q. Sun, J. Su, et al., Org. Lett. 11 (2009) 4918-4921;
(d) Y.F. Kang, L.Y. Niu, Q.Z. Yang, Chin. Chem. Lett. 30 (2019) 1791-1798;
(e) L. Yang, H.Q. Xiong, Y.N. Su, et al., Chin. Chem. Lett. 30 (2019) 563-565;
(f) Y. Yang, H. Wang, Y.L. Wei, J. Zhou, et al., Chin. Chem. Lett. 28 (2017) 2023-2026;
(g) M.Y. Li, P.C. Cui, K. Li, et al., Chin. Chem. Lett. 29 (2018) 992-994;
(h) S. Lee, J. Li, X. Zhou, J. Yin, J. Yoon, Coord. Chem. Rev. 366 (2018) 29-68;
(i) J.C. Xu, H.Q. Yuan, L.T. Zeng, G.M. Bao, Chin. Chem. Lett. 29 (2018) 1456-1464;
(j) C.X. Yin, K.M. Xiong, F.J. Huo, J.C. Salamanca, R.M. Strongin, Angew. Chem. Int. Ed. 56 (2017) 13188-13198;
(k) L.Y. Niu, Y.Z. Chen, H.R. Zheng, et al., Chem. Soc. Rev. 44 (2015) 6143-6160.
-
[5]
(a) L.Y. Niu, Y.S. Guan, Y.Z. Chen, et al., J. Am. Chem. Soc. 134 (2012) 18928-18931;
(b) J. Liu, Y.Q. Sun, Y.Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574-577;
(c) Y.K. Yue, F.J. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181-3185;
(d) K. Umezawa, M. Yoshida, M. Kamiya, T. Yamasoba, Y. Urano, Nat. Chem. 9 (2017) 279-286;
(e) H.H. Song, Y.M. Zhou, H.N. Qu, et al., Ind. Eng. Chem. Res. 57 (2018) 15216-15223;
(f) S.Y. Lim, K.H. Hong, D.I. Kim, H. Kwon, H.J. Kim, J. Am. Chem. Soc. 136 (2014) 7018-7025;
(g) J. Yin, Y. Kwon, D. Kim, et al., J. Am. Chem. Soc. 136 (2014) 5351-5358;
(h) M.H. Lee, J.H. Han, P.S. Kwon, et al., J. Am. Chem. Soc.134 (2012) 1316-1322;
(i) G.X. Yin, T.T. Niu, T. Yu, et al., Angew. Chem. Int. Ed. 58 (2019) 4557-4561;
(j) B. Tang, Y.L. Xing, P. Li, et al., J. Am. Chem. Soc. 129 (2007) 11666-11667;
(k) T.B. Ren, Q.L. Zhang, D.D. Su, et al., Chem. Sci. 9 (2018) 5461-5466;
(l) L.W. He, X.L. Yang, K.X. Xu, X.Q. Kong, W.Y. Lin, Chem. Sci. 8 (2017) 6257-6265.
-
[6]
(a) Z.Q. Xu, X.T. Huang, M.X. Zhang, et al., Anal. Chem. 91 (2019) 11343-11348;
(b) Z.Q. Xu, M.X. Zhang, G.J. Li, et al., Dyes Pigm. 171 (2019)107685;
(c) X. Han, Y.H. Liu, G.T. Liu, et al., Chem. Asian J. 14 (2019) 890-895;
(d) Z.Q. Xu, X.T. Huang, X. Han, et al., Chem 7 (2018) 1609-1628;
(e) G. Liu, X. Han, J. Zhang, et al., Dyes Pigm. 148 (2018) 292-297;
(f) G.T. Liu, W.J. Chen, Z.Q. Xu, Org. Biomol. Chem. 16 (2018) 5517-5523;
(g) M.J. Cao, H.Y. Chen, D. Chen, et al., Chem. Commun. 52 (2016) 721-724.
-
[7]
(a) J. Liu, Y.Q. Sun, H.X. Zhang, et al., Chem. Sci. 5 (2014) 3183-3188;
(b) D.H. Ma, D. Kim, E. Seo, S.J. Lee, K.H. Ahn, Analyst 140 (2015) 422-427;
(c) F.Y. Wang, L. Zhou, C.C. Zhao, et al., Chem. Sci. 6 (2015) 2584-2589;
(d) L. Song, Q. Sun, N. Wang, et al., Anal. Methods 7 (2015) 10371-10375;
(e) D. Lee, G. Kim, J. Yin, J. Yoon, Chem. Commun. 51 (2015) 6518-6520;
(f) C.Y. Zhang, S. Wu, Z. Xi, L. Yi, Tetrahedron 73 (2017) 6651-6656;
(g) D. Lee, K. Jeong, X. Luo, et al., J. Mater. Chem. B 6 (2018) 2541-2546;
(h) S.G. Wang, H.H. Yin, Y. Huang, X.M. Guan, Anal. Chem. 90 (2018) 8170-8177;
(i) J.M. Wang, L.Q. Niu, J. Huang, et al., Dyes Pigm. 158 (2018) 151-156;
(j) J.H. Gao, Y.F. Tao, J. Zhang, et al., Chem. Eur. J. 25 (2019) 11246-11256.
-
[8]
(a) X.L. Sheng, D. Chen, M.J. Cao, et al., Chin. J. Chem. 34 (2016) 594-598;
(b) Z.Q. Xu, M.X. Zhang, Y. Xu, et al., Sens. Actuators B 290 (2019) 676-683.
-
[9]
Y.J. Jiang, J. Cheng, C.Y. Yang, et al., Chem. Sci. 8(2017) 8012-8018.
doi: 10.1039/C7SC03338A
-
[10]
L.Y. Niu, H.R. Zheng, Y.Z. Chen, et al., Analyst 139(2014) 1389-1395.
doi: 10.1039/c3an01849k
-
[11]
B.C. Zhu, M. Zhang, L. Wu, et al., Sens. Actuators B 257(2018) 436-441.
doi: 10.1016/j.snb.2017.10.170

 Login In
Login In






 DownLoad:
DownLoad: