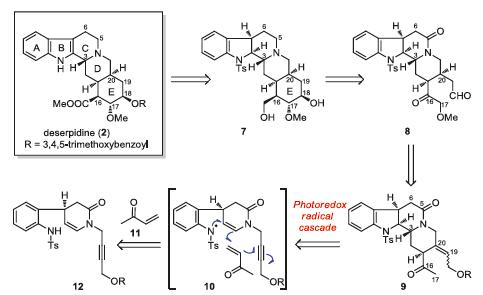
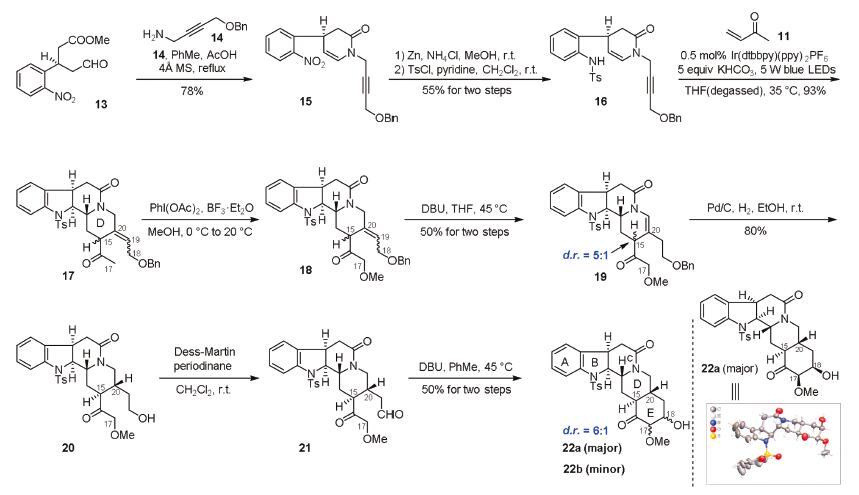
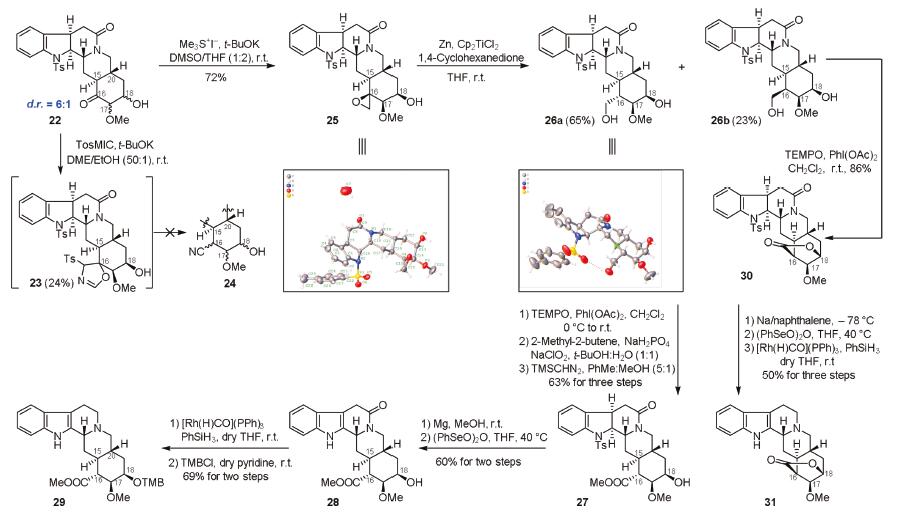
-
[1]
R.E. Woodson, H.W. Younken, E. Schlitter, J.A. Schneider, Rauwolfia: Botany, Pharmacognosy, ChemistryandPharmacology, Little, Brown andCo., Boston, 1957.
-
[2]
(a) J.M. Müller, E. Schlittler, H.J. Bein, Experientia 8 (1952) 338;
(b) E. Schlittler, P.R. Ulshafer, M.L. Pandow, R. Hunt, L. Dorfman, Experientia 11 (1955) 64-65.
-
[3]
(a) H.B. MacPhillamy, C.F. Huebner, E. Schlittler, A.F.St. André, P.R.J. Ulshafer, J. Am. Chem. Soc. 77 (1955) 4335-4343;
(b) P.E. Aldrich, P.A. Diassi, D.F. Dickel, J. Am. Chem. Soc. 81 (1959) 2481-2494;
(c) E. Smith, R.S. Jaret, M. Shamma, R.J. Shine, J. Am. Chem. Soc. 86 (1964) 2083-2084;
(d) M. Lounasmaa, A. Tolvanen, Heterocycles 23 (1985) 371-375.
-
[4]
(a) E. Wenkert, L.H. Liu, D.B.R. Johnston, J. Org, Chem. 30 (1965) 722-728;
(b) S. Takano, F. Ito, K. Ogasawara, Heterocycles 14 (1980) 453-456;
(c) T. Suzuki, A. Tomino, K. Unno, T. Kametani, Chem. Pharm. Bull. Jpn. 29 (1981) 76-81;
(d) S. Chao, F.A. Kunng, J.M. Gu, H.L. Ammon, P.S. Mariano, J. Org. Chem. 49 (1984) 2708-2711;
(e) E. Wenkert, Heterocycles 21 (1984) 325-329;
(f) M.E. Jung, L.A. Light, J. Am. Chem. Soc. 106 (1984) 7614-7618;
(g) M. Isobe, N. Fukami, T. Nishikawa, T. Goto, Heterocycles 25 (1987) 521-532;
(h) R.P. Polniaszek, R.V. Stevens, J. Org. Chem. 51 (1986) 3023-3027.
-
[5]
(a) R.B. Woodward, F.E. Bader, H. Bickel, A.J. Frey, R.W. Kierstead, J. Am. Chem. Soc. 78 (1956) 2023-2025;
(b) R.B. Woodward, F.E. Bader, H. Bickel, A.J. Frey, R.W. Kierstead, Tetrahedron 2 (1958) 1-57;
(c) B.A. Pearlman, J. Am. Chem. Soc. 101 (1979) 6404-6408;
(d) P.A. Wender, J.M. Schaus, A.W. White, J. Am. Chem. Soc. 102 (1980) 6157-6159;
(e) P.A. Wender, J.M. Schaus, A.W. White, Heterocycles 25 (1987) 263-270;
(f) S.F. Martin, H. Rueger, S.A. Williamson, S. Grzejszczak, J. Am. Chem. Soc. 109 (1987) 6124-6134;
(g) G. Stork, Pure Appl. Chem. 61 (1989) 439-442;
(h) S. Hanessian, J. Pan, A. Carnell, H. Bouchard, L. Lesage, J. Org. Chem. 62 (1997) 465-473;
(i) G. Stork, P.C. Tang, M. Casey, B. Goodman, M. Toyota, J. Am. Chem. Soc. 127 (2005) 16255-16262;
(j) S.M. Sparks, A.J. Gutierrez, K.J. Shea, J. Org. Chem. 68 (2003) 5274-5285;
(k) N.S. Rajapaksa, M.A. McGowan, M. Rienzo, W.N. Jacobsen, Org. Lett. 15 (2013) 706-709;
(l) J. Park, D.Y.K. Chen, Angew. Chem. Int. Ed. 130 (2018) 16384-16388.
-
[6]
(a) C. Szántay, G. Blaskó, K. Honty, et al., Liebigs Ann. Chem. 8 (1983) 1292-1309;
(b) S. Sakai, M. Ogawa, Heterocycles 10 (1978) 67-71;
(c) O. Miyata, Y. Hirata, T. Naito, I. Ninomiya, Heterocycles 22 (1984) 1041-1044;
(d) H. Takeaki, M. Yumiko, Okiko, et al., Chem. Pharmaceu. Bull. 37 (1989) 901-906;
(e) P.S. Mariano, E.W. Baxter, D. Labaree, H.L. Ammon, J. Am. Chem. Soc. 112 (1990) 7682-7692;
(f) G. Varchi, A. Battaglia, C. Samorì, et al., J. Nat. Prod. 68 (2005) 1629-1631.
-
[7]
(a) R.W. Wilkins, W.E. Judson, New Eng. J. Med. 248 (1953) 48-56;
(b) R.J. Vakil, Br. Heart J. 11 (1949) 350-355;
(c) M. Bleuler, W.A. Stoll, Ann. New York Acad. Sci. 61 (1955) 167-173;
(d) M.F. Kossover, A.M. Goldman, CHEST 42 (1962) 170-175;
(e) A.S. Leon, M.S. Belle, M. Halpern, CHEST 42 (1962) 626-634;
(f) D.S. Fabricant, N.R. Farnsworth, Envir. Health Persp. 109 (2001) 69-75.
-
[8]
X.Y. Liu, Y. Qin, Acc. Chem. Res. 52 (2019) 1877-1891.
doi: 10.1021/acs.accounts.9b00246
-
[9]
X. Wang, D. Xia, W. Qin, et al., Chem 2 (2017) 803-816.
doi: 10.1016/j.chempr.2017.04.007
-
[10]
R.M. Moriarty, O. Prakash, M.P. Duncan, R.K. Vaid, J. Org. Chem. 52 (1987) 150-153.
doi: 10.1021/jo00377a027
-
[11]
O.H. Oldenziel, D. van Leusen, A.M. VanLeusen, J. Org. Chem. 42 (1977) 3114-3118.
doi: 10.1021/jo00439a002

 Login In
Login In






 DownLoad:
DownLoad: