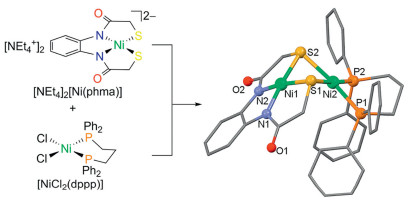
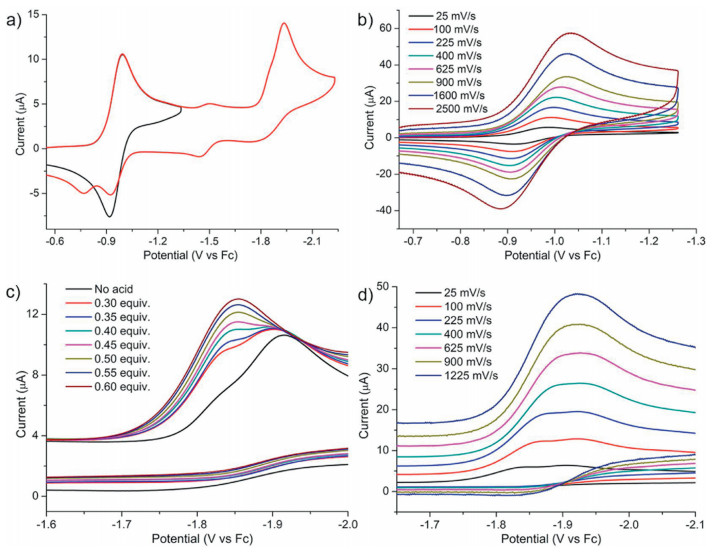
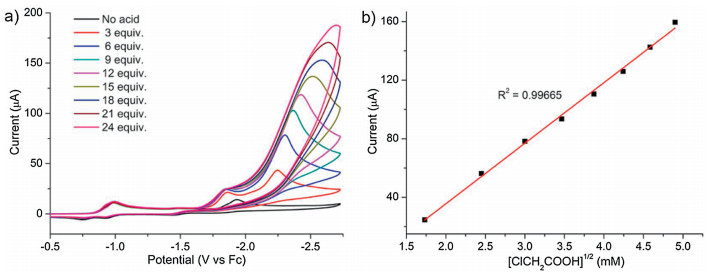
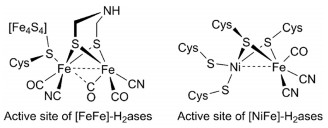
-
[1]
(a) M. Can, F.A. Armstrong, S.W. Ragsdale, Chem. Rev. 114(2014) 4149-4174;
(b) T.R. Simmons, G. Berggren, M. Bacchi, M. Fontecave, V. Artero, Coord. Chem. Rev. 270-271(2014) 127-150;
(c) Y.L. Hu, A.W. Fay, C.C. Lee, J. Yoshizawa, M.W. Ribbe, Biochemistry 47(2008) 3973-3981.
-
[2]
(a) Y.L. Li, T.B. Rauchfuss, Chem. Rev. 116(2016) 7043-7077;
(b) J.F. Capon, F. Gloaguen, F.Y. Pétillon, P. Schollhammer, J. Talarmin, Coord. Chem. Rev. 253(2009) 1476-1494;
(c) I.P. Georgakaki, L.M. Thomson, E.J. Lyon, M.B. Hall, M.Y. Darensbourg, Coord. Chem. Rev. 238-239(2003) 255-266;
(d) N. Wang, M. Wang, L. Chen, L. Sun, Dalton Trans. 42(2013) 12059-12071;
(e) Y. Zhao, X. Yu, H. Hu, et al., Chin. Chem. Lett. 29(2018) 1651-1655.
-
[3]
(a) S. Ogo, Coord. Chem. Rev. 334(2017) 43-53;
(b) D.J. Evans, C.J. Pickett, Chem. Soc. Rev. 32(2003) 268-275;
(c) J.A. Denny, M.Y. Darensbourg, Chem. Rev. 115(2015) 5248-5273;
(d) D. Schilter, J.M. Camara, M.T. Huynh, S. Hammes-Schiffer, T.B. Rauchfuss, Chem. Rev. 116(2016) 8693-8749.
-
[4]
(a) S. Ogo, R. Kabe, K. Uehara, et al., Science 316(2007) 585-587;
(b) S. Ogo, K. Ichikawa, T. Kishima, et al., Science 339(2013) 682-684;
(c) D. Brazzolotto, M. Gennari, N. Queyriaux, et al., Nat. Chem. 8(2016) 1054-1060;
(d) W. Zhu, A.C. Marr, Q. Wang, et al., PNAS 102(2005) 18280-18285;
(e)V.Fourmond, S.Canaguier, B.Golly, etal., EnergyEnviron.Sci.4(2011)2417-2427;
(f) S. Ding, P. Ghosh, A.M. Lunsford, et al., J. Am. Chem. Soc. 138(2016) 12920-12927;
(g) D.H. Manz, P.C. Duan, S. Dechert, et al., J. Am. Chem. Soc. 139(2017) 16720-16731.
-
[5]
(a) D. Brazzolotto, L. Wang, H. Tang, et al., ACS Catal. 8(2018) 10658-10667;
(b) Y.X.C. Goh, H.M. Tang, W.L.J. Loke, W. Fan, Inorg. Chem. 58(2019) 12178-12183;
(c) C.U. Perotto, C.L. Sodipo, G.J. Jones, et al., Inorg. Chem. 57(2018) 2558-2569;
(d) G. Gezer, S. Verbeek, M.A. Sieglerb, E. Bouwman, Dalton Trans. 46(2017) 13590-13596;
(e) B.E. Barton, C.M. Whaley, T.B. Rauchfuss, D.L. Gray, J. Am. Chem. Soc. 131(2009) 6942-6943;
(f) L. Song, Y. Lu, L. Zhu, Q.L. Li, Organometallics 36(2017) 750-760;
(g) P. Sun, D. Yang, Y. Li, et al., Organometallics 35(2016) 751-757;
(h) O.A. Ulloa, M.T. Huynh, C.P. Richers, et al., J. Am. Chem. Soc. 138(2016) 9234-9245;
(i) K. Weber, T. Krämer, H.S. Shafaat, et al., J. Am. Chem. Soc. 134(2012) 20745-20755;
(j) X. Chu, J. Jin, B. Ming, et al., Chem. Sci. 10(2019) 761-767.
-
[6]
(a) M.A. Turner, W.L. Driessen, J. Reedijk, Inorg. Chem. 29(1990) 3331-3335;
(b) P.V. Rao, S. Bhaduri, J. Jiang, R.H. Holm, Inorg. Chem. 43(2004) 5833-5849.
-
[7]
(a) N. Wang, M. Wang, Y. Wang, et al., J. Am. Chem. Soc. 135(2013) 13688-13691;
(b) D. Zheng, N. Wang, M. Wang, et al., J. Am. Chem. Soc. 136(2014) 16817-16823;
(c) D. Li, C.N. Lin, S.Z. Zhan, C.L. Ni, Chin. Chem. Lett. 28(2017) 1424-1428.
-
[8]
A.J. Bard, L.R. Faulkner, Electrochemical Methods:Fundamentals and Applications, 2nd ed., Wiley, New York, 2001.
-
[9]
(a) S.E. Duff, J.E. Barclay, S.C. Davies, D.J. Evans, Inorg. Chem. Commun. 8(2005) 170-173;
(b) D.J. Evans, Eur. J. Inorg. Chem. 22(2005) 4527-4532.
-
[10]
Ø. Hatlevik, M.C. Blanksma, V. Mathrubootham, A.M. Arif, E.L. Hegg, J. Biol. Inorg. Chem. 9(2004) 238-246.
doi: 10.1007/s00775-003-0518-8
-
[11]
G.A.N. Felton, R.S. Glass, D.L. Lichtenberger, D.H. Evans, Inorg. Chem. 45(2006) 9181-9184.
doi: 10.1021/ic060984e
-
[12]
(a) J.M. Savéant, K.B. Su, J. Electroanal. Chem. 191(1984) 341-349;
(b) E.S. Rountree, B.D. McCarthy, T.T. Eisenhart, J.L. Dempsey, Inorg. Chem. 53(2014) 9983-10002;
(c) K.J. Lee, B.D. McCarthy, E.S. Rountree, J.L. Dempsey, Inorg. Chem. 56(2017) 1988-1998.
-
[13]
A.M. Appel, M.L. Helm, ACS Catal. 4(2014) 630-633.
doi: 10.1021/cs401013v
-
[14]
C. Costentin, J.M. Savéant, Chem. Electro. Chem. 1(2014) 1226-1236.
-
[15]
C. Costentin, S. Drouet, M. Robert, J.M. Savéant, J. Am. Chem. Soc. 134(2012) 11235-11242.
doi: 10.1021/ja303560c
-
[16]
C.P. Yap, K. Hou, A.A. Bengali, W.Y. Fan, Inorg. Chem. 56(2017) 10926-10931.
doi: 10.1021/acs.inorgchem.7b01079
-
[17]
(a) M.L. Helm, M.P. Stewart, R.M. Bullock, M.R. DuBois, D.L. DuBois, Science 333(2011) 863-866;
(b) R. Tatematsu, T. Inomata, T. Ozawa, H. Masuda, Angew. Chem. Int. Ed. 55(2016) 5247-5250.

 Login In
Login In






 DownLoad:
DownLoad: