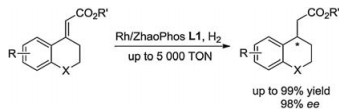
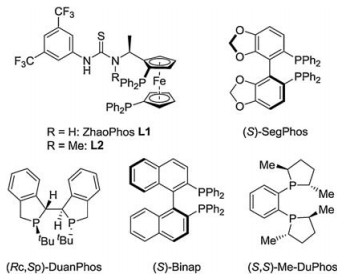
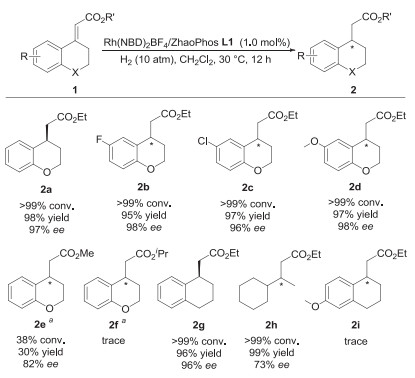
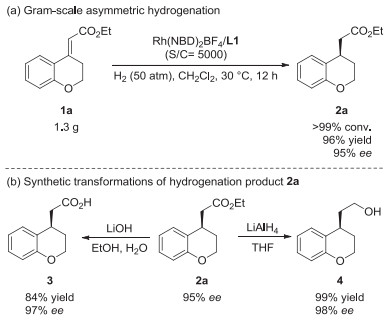
-
[1]
(a) G.R. Geen, J.M. Evans, A.K. Vong, Pyrans and their benzo derivatives: applications, in: A.R. Katritzky, C.W. Rees, E.F.V. Scriven (Eds.), Comprehensive Heterocyclic Chemistry Ⅱ, Vol. 5, PergamonPress, Oxford, UK, 1996, pp.469-500;
(b) D.A. Horton, G.T. Boume, M.L. Smythe, Chem. Rev. 103 (2003) 893-930;
(c) G.P. Ellis, Chromenes, chromanones, and chromones, in: A. Weissberger, E.C. Taylor (Eds.), The Chemistry of Heterocyclic Compounds, Vol. 31, Wiley-VCH, New York, 2007, pp. 1-1196;
(d) K. Tatsuta, T. Tamura, T. Mase, Tetrahedron Lett. 40 (1999) 1925-1928;
(e) Y. Kashiwada, K. Yamazaki, Y. Ikeshiro, et al., Tetrahedron 57 (2001) 1559-1563;
(f) D.J. Maloney, J.Z. Deng, S.R. Starck, Z. Gao, S.M. Hecht, J. Am. Chem. Soc. 127 (2005) 4140-4141;
(g) J.Z. Deng, S.R. Starck, S. Li, S.M. Hecht, J. Nat. Prod. 68 (2005) 1625-1628;
(h) D.J. Maloney, S. Chen, S.M. Hecht, Org. Lett. 8 (2006) 1925-1927;
(i) K.S. Choi, S.G. Kim, Tetrahedron Lett. 51 (2010) 5203-5206;
(j) A.L. Lawrence, R.M. Adlington, E. Jack, et al., Org. Lett. 12 (2010) 1676-1679;
(k) J.P. Lumb, J.L. Krinsky, D. Trauner, Org. Lett. 12 (2010) 5162-5365;
(l) J.T.J. Spence, J.H. George, Org. Lett. 13 (2011) 5318-5321;
(m) S.B. Bharate, R. Mudududdla, J.B. Bharate, et al., Org. Biomol. Chem. 10 (2012) 5143-5150;
(n) J. Liu, X. Wang, L. Xu, et al., Tetrohedron 72 (2016) 7642-7649.
-
[2]
(a) R. Ukis, C. Schneider, J. Org. Chem. 84 (2019) 7175-7188;
(b) A.B. Xia, C. Wu, T. Wang, et al., Adv. Synth. Catal. 356 (2014) 1753-1760;
(c) Z. Wu, S.D. Laffoon, K.L. Hull, Nat. Commun. 9 (2018) 1185-1192.
-
[3]
(a) R. Maity, S.C. Pan, Org. Biomol. Chem. 16 (2018) 1598-1608;
(b) Y. Lee, S.W. Seo, S.G. Kim, Adv. Synth. Catal. 353 (2011) 2671-2675;
(c) K.S. Choi, S.G. Kim, Eur. J. Org. Chem. (2012) 1119-1122;
(d) L. Zu, S. Zhang, H. Xie, W. Wang, Org. Lett. 11 (2009) 1627-1630.
-
[4]
(a) D. Enders, C. Wang, X. Yang, G. Raabe, Adv. Synth. Catal. 352 (2010) 2869-2874;
(b) D. Enders, X. Yang, C. Wang, G. Raabe, J. Runsik, Chem. -Asian J. 6 (2011) 2255-2259;
(c) B.C. Hong, P. Kotame, J.H. Liao, Org. Biomol. Chem. 9 (2011) 382-386;
(d) D.B. Ramachary, M.S. Prasad, R. Madhavachary, Org. Biomol. Chem. 9 (2011) 2715-2721;
(e) S. Jakkampudi, R. Parella, J.C.G. Zhao, Org. Biomol. Chem. 17 (2019) 151-155;
(f) D.B. Ramachary, R. Sakthidevi, K.S. Shruthi, Chem. -Eur. J. 18 (2012) 8008-8012;
(g) D.B. Ramachary, P.S. Reddy, M.S. Prasad, Eur. J. Org. Chem. (2014) 3076-3081;
(h) D. Lu, Y. Li, Y. Gong, J. Org. Chem. 75 (2010) 6900-6907;
(i) J. Yang, G. Qiu, J. Jiang, et al., Adv. Synth. Catal. 359 (2017) 2184-2190.
-
[5]
(a) Z. Wang, J. Sun, Org. Lett. 19 (2017) 2334-2337;
(b) C.C. Hsiao, S. Raja, H.H. Liao, I. Atodiresei, M. Rueping, Angew. Chem. Int. Ed. 54 (2015) 5762-5765.
-
[6]
(a) A. Song, X. Zhang, X. Song, et al., Angew. Chem. Int. Ed. 53 (2014) 4940-4944;
(b) M. Spanka, C. Schneider, Org. Lett. 20 (2018) 4769-4772.
-
[7]
G. Blay, M.C. Muñoz, J.R. Pedro, A.S. Marco, Adv. Synth. Catal. 355(2013) 1071-1076.
doi: 10.1002/adsc.201201120
-
[8]
D.A. Glazier, J.M. Schroeder, J. Liu, W. Tang, Adv. Synth. Catal. 360(2018) 4646-4649.
doi: 10.1002/adsc.201800994
-
[9]
W. Yang, Y. Liu, S. Zhang, Q. Cai, Angew. Chem. Int. Ed. 54(2015) 8805-8808.
doi: 10.1002/anie.201503882
-
[10]
R.M. Neyyappadath, D.B. Cordes, A.M.Z. Slawin, A.D. Smith, Chem. Commun. 53(2017) 2555-2558.
doi: 10.1039/C6CC10178J
-
[11]
Q.G. Wang, S.F. Zhu, L.W. Ye, et al., Adv. Synth. Catal. 352(2010) 1914-1919.
doi: 10.1002/adsc.201000129
-
[12]
K. Fukamizu, Y. Miyake, Y. Nishibayashi, J. Am. Chem. Soc. 130(2008) 10498-10499.
doi: 10.1021/ja8038745
-
[13]
(a) Y. Zhou, J.S. Bandar, R.Y. Liu, S.L. Buchwald, J. Am. Chem. Soc. 140 (2018) 606-609;
(b) V. Hornillos, A.W. Zijl, B.L. Feringa, Chem. Commun. 48 (2012) 3712-3714;
(c) R.C. Carmona, O.D. Köster, C.R.D. Correia, Angew. Chem. Int. Ed. 57 (2018) 12067-12070;
(d) M. Wang, J. Chen, Z. Chen, C. Zhong, P. Lu, Angew. Chem. Int. Ed. 57 (2018) 2707-2711.
-
[14]
(a) W.S. Knowles, Acc. Chem. Res. 16 (1983) 106-112;
(b) R. Noyori, H. Takaya, Acc. Chem. Res. 23 (1990) 345-350;
(c) R. Noyori, T. Ohkuma, Angew. Chem. Int. Ed. 40 (2001) 40-73;
(d) W. Tang, X. Zhang, Chem. Rev. 103 (2003) 3029-3070;
(e) H.U. Blaser, C. Malan, B. Pugin, et al., Adv. Synth. Catal. 345 (2003) 103-151;
(f) X. Cui, K. Burgess, Chem. Rev. 105 (2005) 3272-3296;
(g) A.J. Minnaard, B.L. Feringa, L. Lefort, J.G. De Vries, Acc. Chem. Res. 40 (2007) 1267-1277;
(h) W. Zhang, Y. Chi, X. Zhang, Acc. Chem. Res. 40 (2007) 1278-1290;
(i) N.B. Johnson, I.C. Lennon, P.H. Moran, J.A. Ramsden, Acc. Chem. Res. 40 (2007) 1291-1299;
(j) Y.G. Zhou, Acc. Chem. Res. 40 (2007) 1357-1366;
(k) S.J. Roseblade, A. Pfaltz, Acc. Chem. Res. 40 (2007) 1402-1411;
(l) N. Fleury-Bregeot, V. de la Fuente, S. Castillón, C. Claver, ChemCatChem 2 (2010) 1346-1371;
(m) J.H. Xie, S.F. Zhu, Q.L. Zhou, Chem. Rev. 111 (2011) 1713-1760;
(n) D.S. Wang, Q.A. Chen, S.M. Lu, Y.G. Zhou, Chem. Rev. 112 (2012) 2557-2590;
(o) J.H. Xie, S.F. Zhu, Q.L. Zhou, Chem. Soc. Rev. 41 (2012) 4126-4139;
(p) Q.A. Chen, Z.S. Ye, Y. Duan, Y.G. Zhou, Chem. Soc. Rev. 42 (2013) 497-511;
(q) J.J. Verendel, O. Pamies, M. Dieguez, P.G. Andersson, Chem. Rev. 114 (2014) 2130-2169;
(r) Z. Zhang, N.A. Butt, W. Zhang, Chem. Rev. 116 (2016) 14769-14827.
-
[15]
(a) J. Xia, Y. Nie, G. Yang, Y. Liu, W. Zhang, Org. Lett. 19 (2017) 4884-4887;
(b) B. Qu, L.P. Samankumara, S. Ma, et al., Angew. Chem. Int. Ed. 53 (2014) 14428-14432.
-
[16]
(a) S. Guo, J. (Steve) Zhou, Org. Lett. 18 (2016) 5344-5347;
(b) S. Guo, P. Yang, J. (Steve) Zhou, Chem. Commun. 51 (2015) 12115-12117.
-
[17]
(a) Q. Zhao, S. Li, K. Huang, R. Wang, X. Zhang, Org. Lett. 15 (2013) 4014-4017;
(b) P. Li, M. Zhou, Q. Zhao, et al., Org. Lett. 18 (2016) 40-43;
(c) Q. Zhao, J. Wen, R. Tan, et al., Angew. Chem. Int. Ed. 53 (2014) 8467-8470;
(d) J. Wen, R. Tan, S. Liu, Q. Zhao, X. Zhang, Chem. Sci. 7 (2016) 3047-3051;
(e) Z. Han, P. Li, Z. Zhang, et al., ACS Catal. 6 (2016) 6214-6218;
(f) T. Zhang, J. Jiang, L. Yao, H. Geng, X. Zhang, Chem. Commun. 53 (2017) 9258-9261;
(g) G. Liu, Z. Han, X.Q. Dong, X. Zhang, Org. Lett. 20 (2018) 5636-5639.
-
[18]
(a) B.D. Gallagher, B.R. Taft, B.H. Lipshutz, Org. Lett. 11 (2009) 5374-5377;
(b) F. Song, S. Lu, J. Gunnet, et al., J. Med. Chem. 50 (2007) 2807-2817.

 Login In
Login In






 DownLoad:
DownLoad: