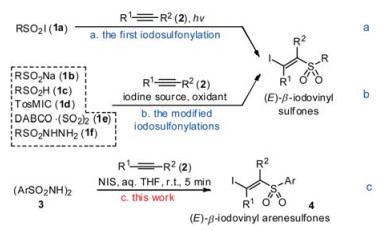
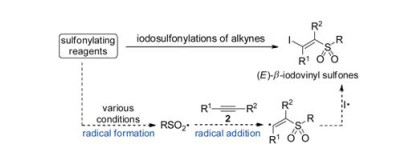
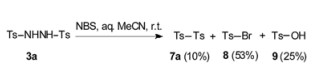
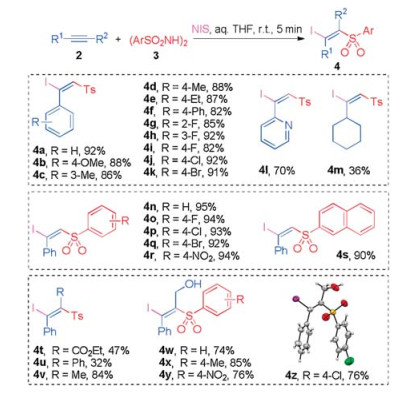
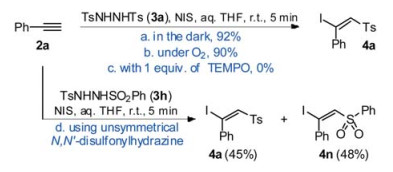
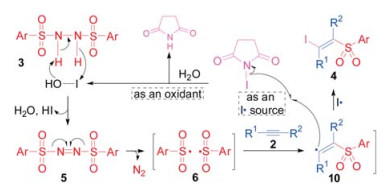
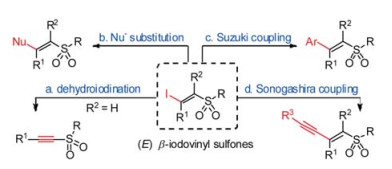
-
[1]
(a) T.G. Back, K.N. Clary, D. Gao, Chem. Rev. 110 (2010) 4498-4553;
(b) M. Nielsen, C.B. Jacobsen, N. Holub, et al., Angew. Chem. Int. Ed. 49 (2010) 2668-2679;
(c) Q. Zhu, Y. Lu, Aust. J. Chem. 62 (2009) 951-955;
(d) T. Pathak, Tetrahedron 64 (2008) 3605-3628;
(e) P.L. Fuchs, T.F. Braish, Chem. Rev. 86 (1986) 903-917.
-
[2]
(a) Y. Fang, Z. Luo, X. Xu, RSC Adv. 6 (2016) 59661-59676;
(b) N.W. Liu, S. Liang, G. Manolikakes, Synthesis 48 (2016) 1939-1973;
(c) D.C. Meadows, J. Gervay-Hague, Med. Res. Rev. 26 (2006) 793-814;
(d) N.S. Simpkins, Tetrahedron 46 (1990) 6951-6984.
-
[3]
(a) J. Jia, Y.A. Ho, R.F. Bulow, et al., Chem. -Eur. J. 24 (2018) 14054-14058;
(b) M.A. Shameem, K. Esfandiarfard, E. Öberg, et al., Chem. -Eur. J. 22 (2016) 10614-10619;
(c) J.R. Bull, N.S. Desmond-Smith, S.J. Heggie, et al., Synlett (1998) 900-902;
(d) N. Iwata, T. Morioka, T. Kobayashi, et al., Bull. Inomata, Chem. Soc. Jpn. 65 (1992) 1379-1388;
(e) W.E. Truce, G.C. Wolf, J. Org. Chem. 36 (1971) 1727-1732.
-
[4]
(a) Y. Liang, S.H. Suzol, Z. Wen, et al., Org. Lett. 18 (2016) 1418-1421;
(b) T. Zoller, D. Uguen, Eur. J. Org. Chem. (1999) 1545-1550;
(c) A. Padwa, D.J. Austin, M. Ishida, et al., J. Org. Chem. 57 (1992) 1161-1169;
(d) W.E. Truce, A.W. Borel, P.J. Marek, J. Org. Chem. 41 (1976) 401-402.
-
[5]
(a) J. Zhang, Z. Liang, J. Wang, et al., ACS Omega 3 (2018) 18002-18015;
(b) Y. Sun, A. Abdukader, D. Lu, et al., Green Chem. 19 (2017) 1255-1258;
(c) Y. Gao, W. Wu, Y. Huang, et al., Org. Chem. Front. 1 (2014) 361-364.
-
[6]
(a) X.X. Gu, M.H. Xie, X.Y. Zhao, et al., Chin. J. Chem. 26 (2008) 1625-1629;
(b) W. Wei, J. Wen, D. Yang, et al., RSC Adv. 5 (2015) 4416-4419;
(c) J.P. Wan, D. Hu, F. Bai, et al., RSC Adv. 6 (2016) 73132-73135.
-
[7]
(a) N. Taniguchi, Tetrahedron 74 (2018) 1454-1460;
(b) R. Kumar, V. Dwivedi, M.S. Reddy, Adv. Synth. Catal. 359 (2017) 2847-2856;
(c) N. Taniguchi, Tetrahedron 70 (2014) 1984-1990;
(d) T. Sawangphon, P. Katrun, K. Chaisiwamongkhol, et al., Synth. Commun. 43 (2013) 1692-1707;
(e) P. Katrun, S. Chiampanichayakul, K. Korworapan, et al., Eur. J. Org. Chem. (2010) 5633-5641;
(f) V. Nair, A. Augustine, T.D. Suja, Synthesis (2002) 2259-2265;
(g) K.M. Short, C.B. Ziegler Jr., Tetrahedron Lett. 36 (1995) 355-356.
-
[8]
Y. Tan, S. Jia, F. Hu, et al., J. Am. Chem. Soc. 140 (2018) 16893-16898.
doi: 10.1021/jacs.8b09893
-
[9]
L. Kadari, R.K. Palakodety, L.P. Yallapragada, Org. Lett. 19 (2017) 2580-2583.
doi: 10.1021/acs.orglett.7b00896
-
[10]
Y. Xiang, Y. Kuang, J. Wu, Chem. Eur. J. 23 (2017) 6996-6999.
doi: 10.1002/chem.201701465
-
[11]
(a) Y. Ma, K. Wang, D. Zhang, et al., Adv. Synth. Catal. 361 (2019) 597-602;
(b) H. Cui, C. He, D. Yang, et al., Synlett 29 (2018) 830-834;
(c) Y. Hou, L. Zhu, H. Hu, et al., New J. Chem. 42 (2018) 8752-8755;
(d) C. Tong, B. Gan, Y. Yan, et al., Synth. Commun. 47 (2017) 1927-1933;
(e) L. Yang, D. Hu, L. Wei, et al., Phosphorus Sulfur Silicon Relat. Elem. 192 (2017) 1301-1304;
(f) G.C. Senadi, B.C. Guo, W.P. Hu, et al., Chem. Commun. (Camb.) 52 (2016) 11410-11413;
(g) N.J. Victor, J. Gana, K.M. Muraleedharan, Chem. -Eur. J. 21 (2015) 14742-14747;
(h) X. Li, X. Xu, X. Shi, Tetrahedron Lett. 54 (2013) 3071-3074.
-
[12]
Y. Kohara, M. Kobayashi, H. Minato, Bull. Chem. Soc. Jpn. 43 (1970) 2933-2937.
doi: 10.1246/bcsj.43.2933
-
[13]
A.T. Maioli, J.P. Anselme, Tetrahedron Lett. 36 (1995) 1221.
-
[14]
(a) D. Guo, J. Liu, L. Wang, Proc. SPIE 7972, Advances in Resist Materials and Processing Technology XXVIII 79722D, San Jose, California, USA, 2011;
(b) E.A. Bartmann, Synthesis (1993) 490-496.
-
[15]
(a) H.H.C. Lakmal, J.X. Xu, X. Xu, et al., J. Org. Chem. 83 (2018) 9497-9503;
(b) X. Wu, B. Liu, Y. Zhang, et al., Angew. Chem. Int. Ed 55 (2016) 12280-12284;
(c) U. Ragnarsson, L. Grehn, Tetrahedron Lett. 53 (2012) 1045-1047;
(d) M. Fernández, E. Reyes, J.L. Vicario, et al., Adv. Synth. Catal. 354 (2012) 371-376;
(e) T. Toma, J. Shimokawa, T. Fukuyama, Org. Lett. 9 (2007) 3195-3197.
-
[16]
C.R.LeBlond, K.Rossen, F.P.Gortsema, et al., TetrahedronLett.42 (2001)8603-8606.
doi: 10.1016/S0040-4039(01)01863-9
-
[17]
(a) E. Dubost, V. Babin, F. Benoist, et al., Org. Lett. 20 (2018) 6302-6305;
(b) Y.D. Vankar, G. Kumaravel, Tetrahedron Lett. 25 (1984) 233-236.

 Login In
Login In






 DownLoad:
DownLoad: