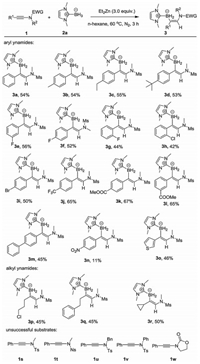
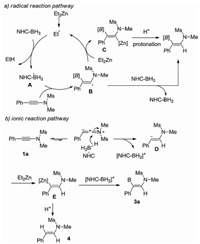
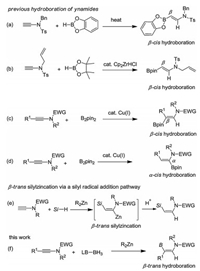
-
[1]
(a) H.C. Brown, Organic Syntheses Via Boranes, Wiley, New York, 1975;
(b) A. Pelter, K. Smith, H.C. Brown, Borane Reagents, Academic Press, New York, 1988;
(c) M. Vaulter, G. Alcaraz, Science of Synthesis, Vinylboranes, Georg Thieme, (2014);
(d) J. Chen, J. Guo, Lu Z, Chin. J. Chem. 36 (2018) 1075-1109.
-
[2]
(a) T. Ohmura, Y. Yamamoto, N. Miyaura, J. Am. Chem. Soc. 122 (2000) 4990-4991;
(b) C. Gunanathan, M. Hçlscher, F. Pan, W. Leitner, J. Am. Chem. Soc. 134 (2012) 14349-14352;
(c) B. Sundararaju, A. Fgrstner, Angew. Chem. Int. Ed. 52 (2013) 14050-14054;
(d) B. Sundararaju, A. Fgrstner, Angew. Chem. 125 (2013) 14300-14304;
(e) Q. Wang, S.E. Motika, N.G. Akhmedov, J.L. Petersen, X. Shi, Angew. Chem. Int. Ed 53 (2014) 5418â€"5422;
(f) Q. Wang, S.E. Motika, N.G. Akhmedov, J.L. Petersen, X. Shi, Angew. Chem.126 (2014) 5522-5526;
(g) J.V. Obligacion, J.M. Neely, A.N. Yazdani, I. Pappas, P.J. Chirik, J. Am. Chem. Soc. 137 (2015) 5855-5858;
(h) W.J. Jang, W.L. Lee, J.H. Moon, J.Y. Lee, J. Yun, Org. Lett.18 (2016) 1390-1393;
(i) S. Xu, Y. Zhang, B. Li, S.Y. Liu, J. Am. Chem. Soc. 138 (2016) 14566-14569;
(j) N. Gorgas, L.G. Alves, B. Stçger, et al., J. Am. Chem. Soc. 139 (2017) 8130-8133;
(k) K. Yuan, N. Suzuki, S.K. Mellerup, et al., Org. Lett. 18 (2016) 720-723;
(l) K. Nagao, A. Yamazaki, H. Ohmiya, M. Sawamura, Org. Lett. 20 (2018) 1861-1865;
(m) J. Guo, B. Cheng, X. Shen, Z. Lu, J. Am. Chem. Soc. 139 (2017) 15316-15319.
-
[3]
(a) K.A. DeKorver, H. Li, A.G. Lohse, et al., Chem. Rev. 110 (2010) 5064-5106;
(b) G. Evano, A. Coste, K. Jouvin, Angew. Chem. Int. Ed. 49 (2010) 2840-2859;
(c) P. Lu, Y. Wang, Chem. Soc. Rev. 41 (2012) 5687-5705;
(d) X.N. Wang, H.S. Yeom, L.C. Fang, et al., Acc. Chem. Res. 47 (2014) 560-578;
(e) A.M. Cook, C. Wolf, Tetrahedron Lett. 56 (2015) 2377-2392;
(f) F. Pan, C. Shu, L.W. Ye, Org. Biomol. Chem. 14 (2016) 9456-9465;
(g) X. Li, Y. Sun, L. Zhang, B. Peng, Chin. J. Org. Chem. 36 (2016) 2530-2544;
(h) G. Duret, V. Le Fouler, P. Bisseret, V. Bizet, N.E. Blanchard, Eur. J. Org. Chem. 46 (2017) 6816-6830;
(i) G. Evano, M. Lecomte, P. Thilmany, C. Theunissen, Synthesis 49 (2017) 3183-3214.
-
[4]
(a) X.L. Han, C.J. Zhou, X.G. Liu, et al., Org. Lett. 19 (2017) 6108-6111;
(b) J. Li, E. Lin, X.L. Han, Q. Li, H. Wang, Org. Lett. 21 (2019) 4255-4258;
(c) P.P. Lin, X.L. Han, G.H. Ye, et al., J. Org. Chem. 84 (2019) 12966-12974.
-
[5]
B. Witulski, N. Buschmann, U. Bergsträßer, Tetrahedron 56(2000) 8473-8480.
doi: 10.1016/S0040-4020(00)00773-0
-
[6]
R.W. Hoffmann, D. Brücknera, New J. Chem. 25(2001) 369-373.
doi: 10.1039/b009259m
-
[7]
Y. Bai, F. Zhang, J. Shen, F. Luo, G. Zhu, Asian J. Org. Chem. 4(2015) 626-629.
doi: 10.1002/ajoc.201500119
-
[8]
G. He, S. Chen, Q. Wang, et al., Org. Biomol. Chem. 12(2014) 5945-5953.
doi: 10.1039/C4OB00979G
-
[9]
J.M. Yang, Z.Q. Li, S.F. Zhu, Chin. J. Org. Chem. 37(2017) 2481-2497.
-
[10]
(a) D.P. Curran, A. Solovyev, M.M. Brahmi, Angew. Chem. Int. Ed. 50 (2011) 10294-10317;
(b) T. Taniguchi, Eur. J. Org. Chem. 37 (2019) 6308-6319;
(c) M. Shimoi, T. Watanabe, K. Maeda, D.P. Curran, T. Taniguchi, Angew. Chem., Int. Ed. 57 (2018) 9485-9490;
(d) W. Dai, S.J. Geib, D.P. Curran, J. Am. Chem. Soc. 141 (2019) 12355-12361;
(e) T. Watanabe, D. Hirose, D.P. Curran, T. Taniguchi, Chem. -Eur. J. 23 (2017) 5404-5409;
(f) J. Qi, F.L. Zhang, Y.S. Huang, et al., Org. Lett. 20 (2018) 2360-2364;
(g) S.C. Ren, F.L. Zhang, J. Qi, et al., J. Am. Chem. Soc. 139 (2017) 6050-6053;
(h) J.K. Jin, F.L. Zhang, Q. Zhao, J.A. Lu, Y.F. Wang, Org. Lett. 20 (2018) 7558-7562;
(i) M. Shimoi, K. Maeda, S.J. Geib, D.P. Curran, T. Taniguchi, Angew. Chem. Int. Ed. 58 (2019) 6357-6361;
(j) S.C. Ren, F.L. Zhang, A.Q. Xu, et al., Nat. Commun. 10 (2019) 1934;
(k) J.K. Jin, W.X. Zheng, H.M. Xia, F.L. Zhang, Y.F. Wang, Org. Lett. 21 (2019) 8414-8418;
(l) X. Liu, E. Lin, G. Chen, et al., Org. Lett. 21 (2019) 8454-8458.
-
[11]
(a) E. Romain, C. Fopp, F. Chemla, et al., Angew. Chem. Int. Ed. 53 (2014) 11333-11337;
(b) K. Vega-Hernandez, E. Romain, A. Coffinet, et al., J. Am. Chem. Soc. 140 (2018) 17632-17642.
-
[12]
(a) X. Pan, E. LacoÌ, te, J. Lalevee, D.P. Curran, J. Am. Chem. Soc.134 (2012) 5669-5674;
(b) C. Chatgilialoglu, Chem. -Eur. J. 14 (2008) 2310-2320.
-
[13]
(a) X. Pan, A. Boussonnière, D.P. Curran, J. Am. Chem. Soc. 135 (2013) 14433-14437;
(b) J.E. Radcliffe, V. Fasano, R.W. Adams, P. Youa, M.J. Ingleson, Chem. Sci. 10 (2019) 1434-1441.
-
[14]
(a) T.S. De Vries, A. Prokofjevs, E. Vedejs, Chem. Rev. 112 (2012) 4246-4282;
(b) A. Solovyev, S.J. Geib, E. LacoÌ, te, D.P. Curran, Organometallics 31 (2012) 54-

 Login In
Login In






 DownLoad:
DownLoad: