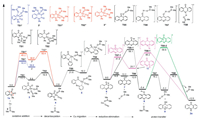
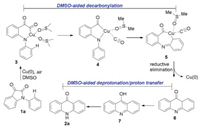
-
[1]
(a) T. Kondo, Y. Taguchi, Y. Kaneko, M. Niimi, T. Mitsudo, Angew. Chem. Int. Ed. 43 (2004) 5369-5372;
(b) P. Chen, T. Xu, G. Dong, Angew. Chem. Int. Ed. 53 (2014) 1674-1678;
(c) T. Kondo, A. Nakamura, T. Okada, et al., J. Am. Chem. Soc. 122 (2000) 6319-6320;
(d) M. Murakami, H. Amii, K. Shigeto, Y. Ito, J. Am. Chem. Soc. 118 (1996) 8285-8290;
(e) R. Zeng, G. Dong, J. Am. Chem. Soc. 137 (2015) 1408-1411.
-
[2]
(a) W. Zhou, Y. Yang, Y. Liu, G.J. Deng, Green Chem. 15 (2013) 76-80;
(b) J. Yu, H. Yang, Y. Jiang, H. Fu, Chem. -Eur. J. 19 (2013) 4271-4277.
-
[3]
H. Wu, Z. Zhang, Q. Liu, et al., Org. Lett. 20(2018) 2897-2901.
doi: 10.1021/acs.orglett.8b00957
-
[4]
(a) I.B. Taraporewala, J.W. Cessac, T.C. Chanh, A.V. Delgado, R.F. Schinazi, J. Med. Chem. 35 (1992) 2744-2752;
(b) O. Tabarrini, G. Manfroni, A. Fravolini, et al., J. Med. Chem. 49 (2006) 2621-2617;
(c) J.X. Kelly, M.J. Smilkstein, R. Brun, et al., Nature 459 (2009) 270-273.
-
[5]
T. Faller, K. Hutton, G. Okafo, et al., Chem. Commun. (1997) 1529-1530.
doi: 10.1039/A701787A
-
[6]
D. Zhang, X. Jiang, H. Yang, et al., Org. Biomol. Chem. 11(2013) 3375-3381.
doi: 10.1039/c3ob27500k
-
[7]
(a) X. Ye, P.N. Plessow, M.K. Brinks, et al., J. Am. Chem. Soc. 136 (2014) 5923-5929;
(b) L.A. Graham, J. Suryadi, T.K. West, G.L. Kucera, U. Bierbach, J. Med. Chem. 55 (2012) 7817-7827.
-
[8]
S.L. MacNeil, B.J. Wilson, V. Snieckus, Org. Lett. 8(2006) 1133-1136.
doi: 10.1021/ol053162e
-
[9]
J. Wen, S. Tang, F. Zhang, R. Shi, A. Lei, Org. Lett. 19(2017) 94-97.
doi: 10.1021/acs.orglett.6b03356
-
[10]
(a) W. Zhou, Y. Liu, Y. Yang, G.J. Deng, Chem. Commun. 48 (2012) 10678-10680;
(b) P.C. Huang, K. Parthasarathy, C.H. Cheng, Chem. -Eur. J. 19 (2013) 460-464.
-
[11]
(a) J. Zhao, R.C. Larock, J. Org. Chem. 72 (2007) 583-588;
(b) X. Pang, Z. Lou, M. Li, L. Wen, C. Chen, Eur. J. Org. Chem. 15 (2015) 3361-3369.
-
[12]
(a) Y. Koguchi, J. Kohno, M. Nishio, et al., J. Antibiot. 53 (2000) 105;
(b) M. Somei, F. Yamada, Nat. Prod. Rep. 20 (2003) 216-242;
(c) C. Jing, T. Shi, D. Xing, X. Guo, W.H. Hu, Green Chem. 15 (2013) 620-624;
(d) E.C. Elliott, E.R. Bowkett, J.L. Maggs, et al., Org. Lett. 20 (2011) 5592-5596;
(e) P. Wu, H. Gao, J. Sun, C.G. Yan, Chin. Chem. Lett. 28 (2017) 329-322;
(f) K. Stratmann, R.E. Moore, R. Bonjouklian, et al., J. Am. Chem. Soc. 116 (1994) 9935-9942;
(g) J.L. Jimenez, U. Huber, R.E. Moore, G.M.L. Patterson, J. Nat. Prod. 62 (1999) 569-572;
(h) H.B. Rasmussen, J.K. Macleod, J. Nat. Prod. 60 (1997) 1152-1154;
(i) T. Tokunaga, W.E. Hume, T. Umezome, et al., J. Med. Chem. 44 (2001) 4641-4649;
(j) Z. Xu, S. Zhang, C. Gao, et al., Chin. Chem. Lett. 28 (2017) 159-167;
(k) R. Shintani, M. Inoue, T. Hayashi, Angew. Chem. Int. Ed. 45 (2006) 3353-3356;
(l) R. Zeng, G. Dong, J. Am. Chem. Soc. 137 (2015) 1408-1411;
(m) K. Meena, S. Kumari, J.M. Khurana, et al., Chin. Chem. Lett. 28 (2017) 136-142;
(n) R.G. Shi, C.G. Yan, Chin. Chem. Lett. 27 (2016) 575-578.
-
[13]
(a) T. Liu, H. Yang, Y. Jiang, H. Fu, Adv. Synth. Catal. 355 (2013) 1169-1176;
(b) G.C. Senadi, W.P. Hu, S.S.K. Boominathan, J.J. Wang, Chem. Eur. J. 21 (2015) 998-1003;
(c) P. Moser, A. Sallmann, I. Wiesenberg, J. Med. Chem. 33 (1990) 2358-2368;
(d) S. Nizalapur, O. Kimyon, N.N. Biswas, et al., Org. Biomol. Chem. 14 (2016) 680-693;
(e) P.C. Huang, P. Gandeepan, C.H. Cheng, Chem. Commun. 49 (2013) 8540-8542;
(f) J. Du, Y. Yang, H. Feng, Y. Li, B. Zhou, Chem. -Eur. J. 20 (2014) 5727-5731;
(g) P. Qian, J.H. Su, Y. Wang, et al., J. Org. Chem. 82 (2017) 6434-6440;
(h) A. Ilangovan, G. Satish, Org. Lett. 15 (2013) 5726-5729;
(i) C. Zhang, S. Li, F. Buress, et al., ACS Catal. 6 (2016) 6853-6860;
(j) Y. Zi, Z.J. Cai, S.Y. Wang, S.J. Ji, Org. Lett. 16 (2014) 3094-3097;
(k) J. Luo, S. Gao, Y. Ma, G. Ge, Synlett 29 (2018) 969-973;
(l) B. Tang, R. Song, C. Wu, et al., J. Am. Chem. Soc. 132 (2010) 8900-8902.
-
[14]
(a) T.X. Liu, S. Yue, C. Wei, et al., Chem. Commun. 54 (2018) 13331-13334;
(b) S. Song, X. Li, X. Sun, Y. Yuan, N. Jiao, Green Chem. 17 (2015) 3285-3289;
(c) Z. Zhang, Q. Tian, J. Qian, et al., J. Org. Chem. 79 (2014) 8182-8188;
(d) J. Qian, Z. Zhang, Q. Liu, T. Liu, G. Zhang, Adv. Synth. Catal. 356 (2014) 3119-3124.
-
[15]
M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013.
-
[16]
(a) Q.C. Zhang, X. Li, X. Wang, et al., Org. Chem. Front. 6 (2019) 679-687;
(b) X. Li, S.J. Li, Y. Wang, et al., Catal. Sci. Technol. 9 (2019) 2514-2522.

 Login In
Login In







 DownLoad:
DownLoad: