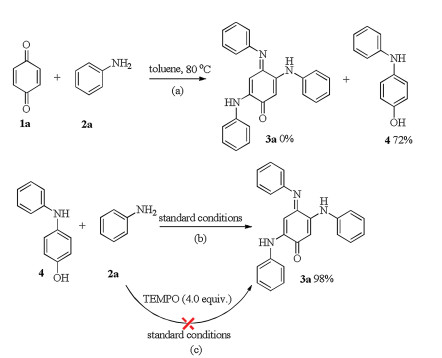
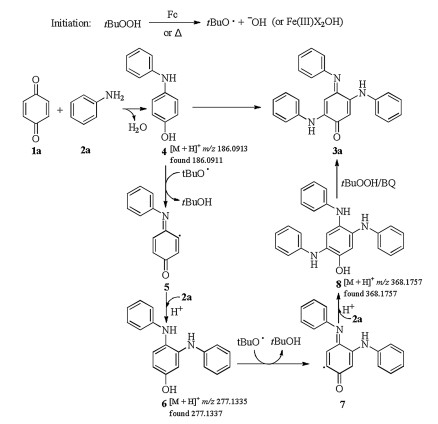
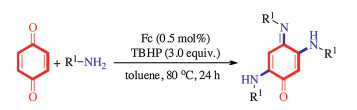
-
[1]
(a) E. Prochazka, B.I. Escher, M.J. Plewa, F.D.L. Leusch, Chem. Res. Toxicol. 28 (2015) 2059-2068;
(b) R. Mout, Z.D. Xu, A.K.H. Wolf, V.J. Davisson, G.K. Jarori, Malar. J.11 (2012) 54-54;
(c) D. Tasdemir, R. Brun, V. Yardley, S.G. Franzblau, P. Ruedi, Chem. Biodiv. 3 (2006) 1230-1237;
(d) L.F. Fieser, E.M. Chamberlin, J. Am. Chem. Soc. 70 (1948) 71-75;
(e) B. Joy, S.N. Kumar, M.S. Soumya, et al., Phytomedicine 21 (2014) 1292-1297;
(f)A.Cavalli, M.L.Bolognesi, S.Capsoni, etal., Angew.Chem.Int.Ed.46 (2007)3689-3692;
(g) T.J. Monks, P. Hanzlik, G.M. Cohen, D. Ross, D.G. Graham, Toxicol. Appl. Pharmacol. 112 (1992) 2-16.
-
[2]
(a) J. Yu, H. Zhang, Q. Lu, et al., Chem. Ind. Eng. Progress 34 (2015) 1115-1121;
(b) M.R. Halhalli, B. Sellergren, Polym. Chem. 6 (2015) 7320-7332;
(c) M.A. Hanna, M.M. Girges, Acta Polym. 41 (1990) 354-360;
(d) S. Rajappa, S.J. Shenoy, Tetrahedron 42 (1986) 5739-5746.
-
[3]
S. Rajappa, R. Sreenivasan, A.V. Rane, Tetrahedron Lett. 24 (1983) 3155-3158.
doi: 10.1016/S0040-4039(00)88121-6
-
[4]
V. Nair, C. Rajesh, R. Dhanya, A.U. Vinod, Tetrahedron Lett. 42 (2001) 2045-2046.
doi: 10.1016/S0040-4039(01)00072-7
-
[5]
V. Nair, R. Dhanya, S. Viji, Tetrahedron 61 (2005) 5843-5848.
doi: 10.1016/j.tet.2005.04.008
-
[6]
K.A. Parker, T.L. Mindt, Org. Lett. 4 (2002) 4265-4268.
doi: 10.1021/ol026849x
-
[7]
(a) Y. Park, Y. Kim, S. Chang, Chem. Rev. 117 (2017) 9247-9301;
(b) J. Kim, S. Chang, Angew. Chem. Int. Ed. 53 (2014) 2203-2207;
(c) T. Kang, Y. Kim, D. Lee, Z. Wang, S. Chang, J. Am. Chem. Soc.136 (2014) 4141-4144;
(d) H. Hwang, J. Kim, J. Jeong, S. Chang, J. Am. Chem. Soc. 136 (2014) 10770-10776;
(e) C. Pi, X. Cui, Y. Wu, J. Org. Chem. 80 (2015) 7333-7339;
(f) M.E. Wei, L.H. Wang, Y.Y. Li, X. Cui, Chin. Chem. Lett. 26 (2015) 1336-1340;
(g) X. Han, P. Lin, Q. Li, Chin. Chem. Lett. 30 (2019) 1495-1502;
(h) S. Yuan, S. Wang, M. Zhao, et al., Chin. Chem. Lett. 31 (2020) 349-352;
(i) Q. Huang, L. Zhu, D. Yi, X. Zhao, W. Wei, Chin. Chem. Lett. 31 (2020) 373-376;
(j) X. Zhang, S. Dong, Q. Ding, X. Fan, G. Zhang, Chin.Chem. Lett. 30 (2019) 375-378;
(k) L. Xie, S. Peng, L. Jiang, et al., Org. Chem. Front. 6 (2019) 167-171;
(l) L. Xie, S. Peng, F. Liu, et al., ACS Sustainable Chem. Eng. 7 (2019) 7193-7199.
-
[8]
J.A. Jordan-Hore, C.C.C. Johansson, M. Gulias, E.M. Beck, M.J. Gaunt, J. Am. Chem. Soc. 130 (2008) 16184-16186.
doi: 10.1021/ja806543s
-
[9]
S.M. Paradine, M.C. White, J. Am. Chem. Soc. 134 (2012) 2036-2039.
doi: 10.1021/ja211600g
-
[10]
(a) Y. Feng, Y. Li, Y. Yu, L. Wang, X. Cui, RSC Adv. 8 (2018) 8450-8454;
(b) Y. Feng, Z. Zhang, Q. Fu, et al., Chin. Chem. Lett. 31 (2020) 58-60.
-
[11]
(a) C. Liu, D. Liu, A. Lei, Acc. Chem. Res. 47 (2014) 3459-3470;
(b) S.A. Girard, T. Knauber, C.J. Li, Angew. Chem. Int. Ed. 53 (2014) 74-100;
(c) R. Braslau, M.O. Anderson, F. Rivera, et al., Tetrahedron 58 (2002) 5513-5523;
(d) S. Bath, N.M. Laso, H. Lopez-Ruiz, B. Quiclet-Sire, S.Z. Zard, Chem. Commun. 34 (2003) 204-205;
(e) J. Wang, C. Liu, J. Yuan, A. Lei, Angew. Chem. Int. Ed. 52 (2013) 2256-2259;
(f) J. Xie, J. Yu, M. Rudolph, F. Rominger, A.S. Hashmi, Angew. Chem. Int. Ed. 55 (2016) 9416-9421;
(g) C. Wang, J. Qin, X. Shen, et al., Angew. Chem. Int. Ed. 55 (2016) 685-688;
(h) L.Y. Xie, S. Peng, F. Liu, et al., Org. Chem. Front. 5 (2018) 2604-2609;
(i) L.Y. Xie, S. Peng, F. Liu, et al., Adv. Synth. Catal. 360 (2018) 4259-4264;
(j) L. Xie, T. Fang, J. Tan, et al., Green Chem. 21 (2019) 3858-3863.
-
[12]
(a) D. Leifert, C.G. Daniliuc, A. Studer, Org. Lett. 15 (2013) 6286-6289;
(b) S. Wertz, D. Leifert, A. Studer, Org. Lett. 15 (2013) 928-931.
-
[13]
Y. Feng, H. Zhang, Y. Yu, L. Yang, X. Cui, Eur. J. Org. Chem. 16 (2019) 2740-2744.
-
[14]
(a) L. Wang, D. Xiong, L. Jie, C. Yu, X. Cui, Chin. Chem. Lett. 29 (2018) 907-910;
(b) L. Xu, T. Li, L. Wang, X. Cui, J. Org. Chem. 84 (2019) 560-567;
(c) Z. Yang, L. Jie, Z. Yao, et al., Adv. Catal. Synth. 1 (2019) 214-258;
(d) P. Chao, X. Yin, X. Cui, Y. Ma, Y. Wu, Org. Lett. 7 (2019) 2081-2084;
(e) J. Ren, C. Pi, Y. Wu, X. Cui, Org. Lett. 11 (2019) 4067-4071; f) S. Huang, H. Li, X. Sun, et al., Org. Lett. 21 (2019) 5570-5574;
(g) B. Wu, Z. Yang, H. Zhang, L. Wang, Cui X, Chem. Commun. 55 (2019) 4190-4193;
(h) Z. Yang, Z. Song, L. Jie, L. Wang, X. Cui, Chem. Commun. 55 (2019) 6094-6097;
(i) T. Yuan, C. Pi, C. You, et al., Chem. Commun. 55 (2019) 163-166;
(k) T. Wan, S. Du, C. Pi, Y. Wang, R. Li, Y. Wu, X. Cui, ChemCatChem 11 (2019) 3791-3796;
(l) S. Du, C. Pi, T. Wan, Y. Wu, X. Cui, Adv. Synth. Catal. 361 (2019) 1766-1770;
(m) Z.H. Shen, C. Pi, X. Cui, Y. Wu, Chin. Chem. Lett. 30 (2019) 1374-1378.

 Login In
Login In






 DownLoad:
DownLoad: