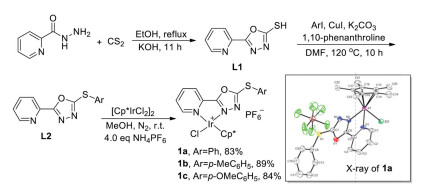
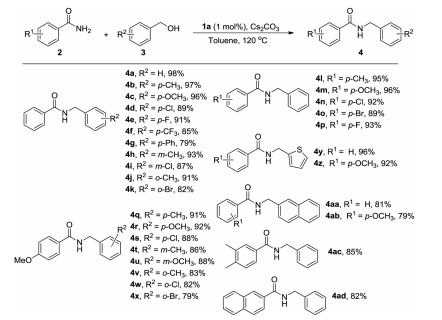
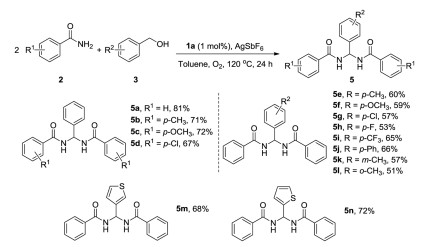
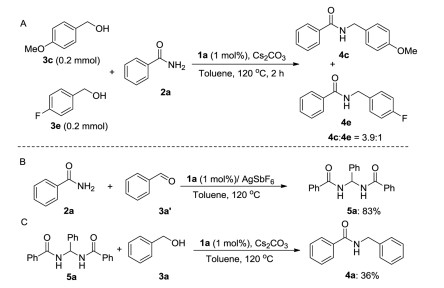
-
[1]
(a) H.R. Shaterian, A. Hosseinian, M. Ghashang, Can. J. Chem. 86 (2008) 376-383;
(b) P. Yang, K.Z. Myint, Q. Tong, et al., J. Med. Chem. 55 (2012) 9973-9987;
(c) C. Ma, L. Wang, P. Yang, K.Z. Myint, X.Q. Xie, J. Chem. Inf. Model. 53 (2013) 11-26.
-
[2]
(a) H.R.Shaterian, M.Ghashang, M.Feyzi, Appl.Catal.AGen.345 (2008)128-133;
(b) J.W. Bode, Curr. Opin. Drug Discov. Devel. 9 (2006) 765-775;
(c) M. Rodriguez, P. Dubreuil, J.P. Bali, J. Martinez, J. Med. Chem. 30 (1987) 758-763;
(d) M.G. Liu, N. Liu, W.H. Xu, L. Wang, Tetrahedron 75 (2019) 2748-2754.
-
[3]
(a) G. Kour, M. Gupta, Dalton Trans. 46 (2017) 7039-7050;
(b) M. Kour, S. Paul, New J. Chem. 39 (2015) 6338-6350;
(c) B. Maleki, M. Baghayeri, RSC Adv. 5 (2015) 79746-79758;
(d) R. Tayebee, B. Maleki, F.M. Zonoz, R.M. Kakhki, T. Kunani, RSC Adv. 6 (2016) 20687-20694;
(e) L. Wang, Y.B. Xie, N.Y. Huang, et al., ACS Catal. 6 (2016) 4010-4016;
(f) L. Wang, Y.B. Xie, N.Y. Huang, et al., Adv. Synth. Catal. 359 (2017) 779-785;
(g) N. Liu, F. Chao, M.G. Liu, et al., J. Org. Chem. 84 (2019) 2366-2371.
-
[4]
(a) A. Corma, J. Navas, M.J. Sabater, Chem. Rev. 118 (2018) 1410-1459;
(b) G. Chelucci, Coord. Chem. Rev. 331 (2017) 1-36;
(c) F. Huang, Z. Liu, Z. Yu, Angew. Chem. Int. Ed. 55 (2016) 862-875;
(d) Q. Yang, Q. Wang, Z. Yu, Chem. Soc. Rev. 44 (2015) 2305-2329;
(e) A. Nandakumar, S.P. Midya, V.G. Landge, E. Balaraman, Angew. Chem. Int. Ed. 54 (2015) 11022-11034;
(f) B. Chen, L. Wang, S. Gao, ACS Catal. 5 (2015) 5851-5876;
(g) K.I. Shimizu, Catal. Sci. Technol. 5 (2015) 1412-1427;
(h) Y. Obora, ACS Catal. 4 (2014) 3972-3981;
(i) D. Hollmann, ChemSusChem 7 (2014) 2411-2413.
-
[5]
(a) A.J.A. Watson, J.M.J. Williams, Science 329 (2010) 635-636;
(b) C. Gunanathan, D. Milstein, Science 341 (2013) 249;
(c) P. Hu, Y. Ben-David, D. Milstein, Angew. Chem. Int. Ed. 55 (2016) 1061-1064;
(d) P. Daw, S. Chakraborty, J.A. Garg, Y. Ben-David, D. Milstein, Angew. Chem. Int. Ed. 55 (2016) 14373-14377;
(e) S. Gowrisankar, H. Neumann, M. Beller, Angew. Chem. Int. Ed. 50 (2011) 5139-5143;
(f) L. Neubert, D. Michalik, S. Bähn, et al., J. Am. Chem. Soc. 134 (2012) 12239-12244;
(g) M. Zhang, X. Fang, H. Neumann, M. Beller, J. Am. Chem. Soc. 135 (2013) 11384-11388;
(h) J. Schranck, A. Tlili, M. Beller, Angew. Chem. Int. Ed. 52 (2013) 7642-7644;
(i) M. Zhang, H. Neumann, M. Beller, Angew. Chem. Int. Ed. 52 (2013) 597-601;
(j) C.S. Lim, T.T. Quach, Y. Zhao, Angew. Chem. Int. Ed. 56 (2017) 7176-7180.
-
[6]
(a) F.G. Mutti, T. Knaus, N.S. Scrutton, M. Breuer, N.J. Turner, Science 349 (2015) 1525-1529;
(b) J.R. Frost, C.B. Cheong, W.M. Akhtar, et al., J. Am. Chem. Soc. 137 (2015) 15664-15667;
(c) N. Deibl, R. Kempe, J. Am. Chem. Soc. 138 (2016) 10786-10789;
(d) T. Yan, B.L. Feringa, K. Barta, ACS Catal. 6 (2016) 381-388;
(e) C. Schlepphorst, B. Maji, F. Glorius, ACS Catal. 6 (2016) 4184-4188;
(f) B. Emayavaramban, M. Roy, B. Sundararaju, Chem. -Eur. J. 22 (2016) 3952-3955;
(g) D. Shen, D.L. Poole, C.C. Shotton, et al., Angew. Chem. Int. Ed. 54 (2015) 1642-1645;
(h) F. Jiang, M. Achard, C. Bruneau, Chem. -Eur. J. 21 (2015) 14319-14323;
(i) M.V. Jimenez, J. Fernandez-Tornos, F.J. Modrego, J.J. Perez-Torrente, L.A. Oro, Chem. -Eur. J. 21 (2015) 17877-17889;
(j) A.J. Rawlings, L.J. Diorazio, M. Wills, Org. Lett. 17 (2015) 1086-1089;
(k) T.T. Dang, B. Ramalingam, A.M. Seayad, ACS Catal. 5 (2015) 4082-4088;
(l) X. Xie, H.V. Huynh, ACS Catal. 5 (2015) 4143-4151;
(m) H. Hikawa, T. Koike, K. Izumi, S. Kikkawa, I. Azumaya, Adv. Synth. Catal. 358 (2016) 784-791;
(n) H. Hikawa, R. Ichinose, S. Kikkawa, I. Azumaya, Green Chem. 20 (2018) 1297-1305.
-
[7]
(a) B. Xiong, S. Zhang, H. Jiang, M. Zhang, Org. Lett. 18 (2016) 724-727;
(b) Z. Tan, H. Jiang, M. Zhang, Org. Lett. 18 (2016) 3174-3177;
(c) B. Xiong, S.D. Zhang, L. Chen, et al., Chem. Commun. 52 (2016) 10636-10639;
(d) Z. Tan, H. Jiang, M. Zhang, Chem. Commun. 52 (2016) 9359-9362;
(e) F. Xie, R. Xie, J.X. Zhang, et al., ACS Catal. 7 (2017) 4780-4785;
(f) H.J. Pan, T.W. Ng, Y. Zhao, Chem. Commun. 51 (2015) 11907-11910;
(g) Z.Q. Rong, Y. Zhang, R.H.B. Chua, H.J. Pan, Y. Zhao, J. Am. Chem. Soc.137 (2015)4944-4947;
(h) Y. Zhang, C.S. Lim, D.S.B. Sim, H.J. Pan, Y. Zhao, Angew. Chem. Int. Ed. 53 (2014) 1399-1403;
(i) C.S. Lim, T.T. Quach, Y. Zhao, Angew. Chem. Int. Ed. 56 (2017) 7176-7180;
(j) X. Chen, H. Zhao, C. Chen, H. Jiang, M. Zhang, Angew. Chem. Int. Ed. 56 (2017) 14232-14326.
-
[8]
(a) F. Li, L. Lu, P. Liu, Org. Lett. 18 (2016) 2580-2583;
(b) R. Wang, H. Fan, W. Zhao, F. Li, Org. Lett. 18 (2016) 3558-3561;
(c) S.Y. Li, X.H. Li, Q. Li, et al., Green Chem. 17 (2015) 3260-3265;
(d) Q. Xu, J. Chen, H. Tian, et al., Angew. Chem. Int. Ed. 53 (2014) 225-229;
(e) X. Cui, X. Dai, Y. Deng, F. Shi, Chem. -Eur. J. 19 (2013) 3665-3675;
(f) Q. Wang, K. Wu, Z. Yu, Organometallics 35 (2016) 1251-1256;
(g) T. Liu, L. Wang, K. Wu, Z. Yu, ACS Catal. 8 (2018) 7201-7207.
-
[9]
(a) C. Gunanathan, D. Milstein, Angew. Chem. Int. Ed. 47 (2008) 8661-8664;
(b) F. Shi, M.K. Tse, X.J. Cui, et al., Angew. Chem. Int. Ed. 48 (2009) 5912-5915;
(c) B. Gnanaprakasam, J. Zhang, D. Milstein, Angew. Chem. Int. Ed. 49 (2010) 1468-1471;
(d) Y. Zhao, S.W. Foo, S. Saito, Angew. Chem. Int. Ed. 50 (2011) 3006-3009;
(e) S. Gowrisankar, H. Neumann, M. Beller, Angew. Chem. Int. Ed. 50 (2011) 5139-5143;
(f)L.Neubert, D.Michalik, S.Bähn, etal., J.Am.Chem.Soc.134 (2012)12239-12244;
(g) T. Yan, B.L.T. Feringa, K. Barta, Nat. Commun. 5 (2014) 5602-560;
(h) T. Yan, B.L. Feringa, K. Barta, ACS Catal. 6 (2016) 381-388;
(i) P. Hu, Y. Ben-David, D. Milstein, Angew. Chem. Int. Ed. 55 (2016) 1061-1064;
(j) N. Deibl, R. Kempe, J. Am. Chem. Soc. 138 (2016) 10786-10789.
-
[10]
(a) X. Cui, Y. Zhang, F. Shi, Y. Deng, Chem. -Eur. J. 17 (2011) 1021-1028;
(b) Q. Xu, H.M. Xie, E.L. Zhang, et al., Green Chem. 18 (2016) 3940-3944;
(c) S. Kerdphon, X. Quan, V.S. Parihar, P.G. Andersson, J. Org. Chem. 80 (2015) 11529-11537;
(d) J. Das, D. Banerjee, J. Org. Chem. 83 (2018) 3378-3384;
(e) G.C.Y. Choo, H. Miyamura, S. Kobayashi, Chem. Sci. 6 (2015) 1719-1727.
-
[11]
(a) D. Wang, K. Zhao, C. Xu, H. Miao, Y. Ding, ACS Catal. 4 (2014) 3910-3918;
(b) Y. Yang, A. Qin, K. Zhao, D. Wang, X. Shi, Adv. Synth. Catal. 358 (2016) 1433-1439;
(c) Z. Xu, D.S. Wang, X. Yu, Y. Yang, D. Wang, Adv. Synth. Catal. 359 (2017) 3332-3340;
(d) Q. Wu, L. Pan, G. Du, C. Zhang, D. Wang, Org. Chem. Front. 5 (2018) 2668-2675;
(e) R. Huang, Y. Yang, D.S. Wang, L. Zhang, D. Wang, Org. Chem. Front. 5 (2018) 203-209;
(f) C. Ge, X. Sang, W. Yao, L. Zhang, D. Wang, Green Chem. 20 (2018) 1805-1812;
(g) Z. Xu, X. Yu, X. Sang, D. Wang, Green Chem. 20 (2018) 2571-2577;
(h) X. Hu, H. Zhu, X. Sang, D. Wang, Adv. Synth. Catal. 360 (2018) 4293-4300;
(j) D. Ye, R. Huang, H. Zhu, L.H. Zou, D. Wang, Org. Chem. Front. 6 (2019) 62-69;
(j.) D. Ye, L. Pan, H. Zhu, et al., Mater.Chem. Front. 3 (2019) 216-223.
-
[12]
D. Wang, R. Cai, S. Sharma, et al., J. Am. Chem. Soc. 134 (2012) 9012-9019.
doi: 10.1021/ja303862z
-
[13]
(a) D. Wang, X. Yu, X. Xu, et al., Chem. -Eur. J. 22 (2016) 8663-8668;
(b) X. Yu, D.S. Wang, Z. Xu, B. Yang, D. Wang, Org. Chem. Front. 4 (2017) 1011-1018;
(c) X. Yu, W. Yao, H. Wan, Z. Xu, D. Wang, J. Organomet. Chem. 822 (2016) 100-103.

 Login In
Login In






 DownLoad:
DownLoad: