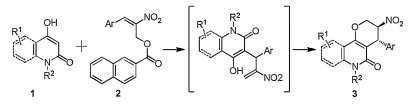
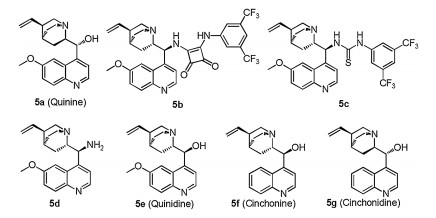
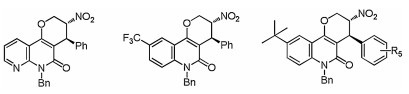
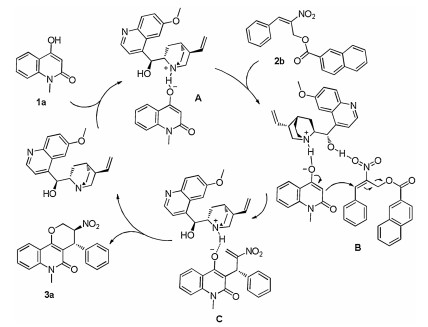
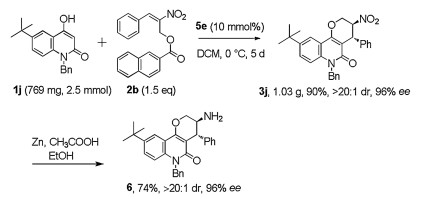
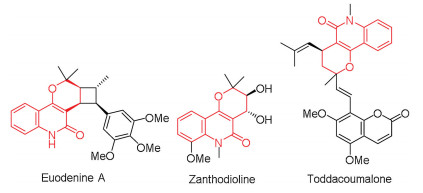
-
[1]
(a) T. Shiro, T. Fukaya, M. Tobe, Eur. J. Med. Chem. 97 (2015) 397-408;
(b) B. Liu, F. Li, T. Zhou, X.Q. Tang, G.W. Hu, J. Heterocycl. Chem. 55 (2018) 1863-1873;
(c) T. Yasumoto, Chem. Rec. 1 (2001) 228-242;
(d) A. Bermejo, B. Figadère, M.C. Zafra-Polo, et al., Nat. Prod. Rep. 22 (2005) 269-303;
(e) M. Murata, T. Yasumoto, Nat. Prod. Rep. 17 (2000) 293-314.
-
[2]
(a) V. Nadaraj, S. Thamarai Selvi, H. Pricilla Bai, S. Mohan, T. Daniel Thangadurai, Med. Chem. Res. 21 (2011) 2902-2910;
(b) Z.D. Yang, D.B. Zhang, J. Ren, M.J. Yang, Med. Chem. Res. 21 (2012) 722-725.
-
[3]
J.E. Neve, H.P. Wijesekera, S. Duffy, et al., J. Med. Chem. 57 (2014) 1252-1275.
doi: 10.1021/jm401321v
-
[4]
I.S. Chen, I.W. Tsai, C.M. Teng, et al., Phytochemistry 46 (1997) 525-529.
doi: 10.1016/S0031-9422(97)00280-X
-
[5]
T.T. Lin, Y.Y. Huang, G.H. Tang, et al., J. Nat. Prod. 77 (2014) 955-962.
doi: 10.1021/np401040d
-
[6]
(a) X.X. Sun, H.H. Zhang, G.H. Li, Y.Y. He, F. Shi, Chem. -Eur. J. 22 (2016) 17526-17532;
(b) Z.Q. Zhu, L. Yu, M. Sun, G.J. Mei, F. Shi, Adv. Synth. Catal. 360 (2018) 3109-3116;
(c) X.X. Sun, C. Li, Y.Y. He, et al., Adv. Synth. Catal. 359 (2017) 2660-2670;
(d) W. Dai, H. Lu, F. Shi, S.J. Tu, Chem. -Eur. J. 20 (2014) 11382-11389;
(e) X. Yang, Y.C. Zhang, Q.N. Zhu, M.S. Tu, F. Shi, J. Org. Chem. 81 (2016) 5056-5065;
(f) F. Shi, R.Y. Zhu, W. Dai, C.S. Wang, S.J. Tu, Chem. -Eur. J. 20 (2014) 2597-2604.
-
[7]
(a) A. Temperini, A. Barattucci, P.M. Bonaccorsi, O. Rosati, L. Minuti, J. Org. Chem. 80 (2015) 8102-8112;
(b) C. Bosset, P. Angibaud, I. Stanfield, et al., J. Org. Chem. 80 (2015) 12509-12525;
(c) T.J. Potter, J.A. Ellman, Org. Lett. 18 (2016) 3838-3841;
(d) J. Scoccia, S.J. Pérez, V. Sinka, et al., Org. Lett. 19 (2017) 4834-4837;
(e) Y. Kurimoto, T. Nasu, Y. Fujii, K. Asano, S. Matsubara, Org. Lett. 21 (2019) 2156-2160;
(f) X. Xie, C. Peng, G. He, et al., Chem. Commun. 48 (2012) 10487-10489.
-
[8]
(a) J.Y. Liu, X.C. Yang, H. Lu, Y.C. Gu, P.F. Xu, Org. Lett. 20 (2018) 2190-2194;
(b) D.K. Nair, R.F.S. Menna-Barreto, E.N. da Silva Júnior, S.M. Mobin, I.N.N. Namboothiri, Chem. Commun. 50 (2014) 6973-6976;
(c) R. Chen, X. Fan, J. Gong, Z. He, Asian J. Org. Chem. 3 (2014) 877-885;
(d) C.Y. Yu, M. Yaqub, Y.M. Jia, Z.T. Huang, Synlett 9 (2008) 1357-1360;
(e) W. Xiao, X. Yin, Z. Zhou, W. Du, Y.C. Chen, Org. Lett. 18 (2015) 116-119;
(f) S. Chandrasekhar, K. Mallikarjun, G. Pavankumarreddy, K.V. Rao, B. Jagadeesh, Chem. Commun. (2009) 4985-4987;
(g) B. Han, Y.C. Xiao, Z.Q. He, Y.C. Chen, Org. Lett. 11 (2009) 4660-4663;
(h) M.L. Shi, G. Zhan, S.L. Zhou, W. Du, Y.C. Chen, Org. Lett. 18 (2016) 6480-6483;
(i) L. Yang, W. Huang, X.H. He, et al., Adv. Synth. Catal. 358 (2016) 2970-2975.
-
[9]
R. Gurubrahamam, B.F. Gao, Y.M. Chen, et al., Org. Lett. 18 (2016) 3098-3101.
doi: 10.1021/acs.orglett.6b01265
-
[10]
(a) V. Modrocká, E. Veverková, M. Meciarová, R. Šebesta, J. Org. Chem. 83 (2018) 13111-13120;
(b) J. Zhang, G. Yin, Y. Du, et al., J. Org. Chem. 82 (2017) 13594-13601.
-
[11]
Y. Zheng, L. Cui, Y. Wang, Z. Zhou, J. Org. Chem. 81 (2016) 4340-4346.
doi: 10.1021/acs.joc.6b00196
-
[12]
E. Vitaku, D.T. Smith, J.T. Njardarson, J. Med. Chem. 57 (2014) 10257-10274.
doi: 10.1021/jm501100b
-
[13]
(a) V. Nair, P.M. Treesa, C.N. Jayan, et al., Tetrahedron 57 (2001) 7711-7717;
(b) A. Klásek, O. Rudolf, M. Rouchal, A. Lycka, A. Ru 9žicka, Tetrahedron 69 (2013) 9492-499;
(c) Y. Gu, J. Barrault, F. Jérôme, Adv. Synth. Catal. 351 (2009) 3269-3278;
(d) M. Rueping, E. Merino, M. Bolte, Org. Biomol. Chem. 10 (2012) 6201-6210;
(e) Y.R. Lee, B.S. Kim, H.I. Kweon, Tetrahedron 56 (2000) 3867-3874;
(f) Y.R. Lee, H.I. Kweon, W.S. Koh, et al., Synthesis (Stuttgart) (2001) 1851-1855;
(g) X. Zhu, A. Lin, Y. Shi, et al., Org. Lett. 13 (2011) 4382-4385.
-
[14]
(a) W. Xiao, X. Yin, Z. Zhou, W. Du, Y.C. Chen, Org. Lett. 18 (2016) 116-119;
(b) L.F. Yeh, S. Anwar, K. Chen, Tetrahedron 68 (2012) 7317-7321;
(c) D.K. Nair, S.M. Mobin, I.N.N. Namboothiri, Tetrahedron Lett. 53 (2012) 3349-3352.
-
[15]
A. Dey, A. Hajra, Org. Biomol. Chem. 15 (2017) 8084-8090.
doi: 10.1039/C7OB02124K

 Login In
Login In






 DownLoad:
DownLoad: