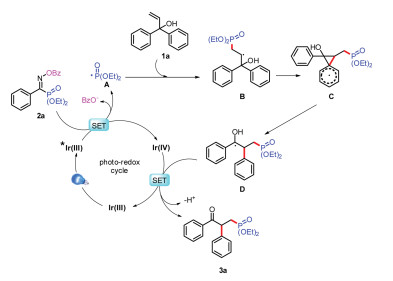
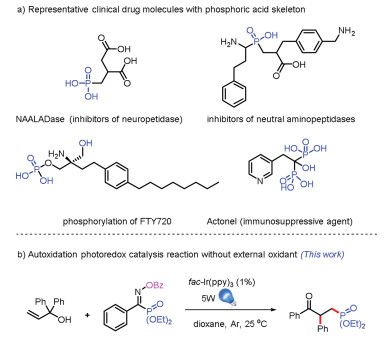
-
[1]
(a) P.J. Murphy, Organophosphorus Reagents, OxfordUniv. Press, Oxford, 2004;
(b) D.T. Kolio, Chemistry and Application of H-phosphonates, Elsevier Science, Oxford, 2006;
(c) N.S. Li, J.K. Frederiksen, J.A. Piccirilli, Acc. Chem. Res. 44 (2011) 1257-1269;
(d) H.H. Chen, L. Zhu, K.B. Zhong, et al., Chin. Chem. Lett. 29 (2018) 1237-1241.
-
[2]
(a) P.F. Jackson, K.L. Tays, K.M. Maclin, et al., J. Med. Chem. 44 (2001) 4170-4175;
(b) R. Albert, K. Hinterding, V. Brinkmann, et al., J. Med. Chem. 48 (2005) 5373-5377;
(c) S. Vassiliou, E.W. Tomczak, L. Berlicki, et al., J. Med. Chem. 57 (2014) 8140-8151;
(d) Z. Novakova, K. Wozniak, A. Jancarik, et al., J. Med. Chem. 59 (2016) 4539-3550;
(e) G.P. Horsman, D.L. Zechel, Chem. Rev. 117 (2017) 5704-5783.
-
[3]
(a) A.L. Schwan, Chem. Soc. Rev. 33 (2004) 218-224;
(b) C.S. Demmer, N.K. Larsen, L. Bunch, Chem. Rev. 111 (2011) 7981-8006;
(c) X.Q. Pan, J.P. Zou, W.B. Yi, W. Zhang, Tetrahedron 71 (2015) 7481-7529;
(d) K. Luo, W.C. Yang, L. Wu, Asian J. Org. Chem. 6 (2017) 350-367;
(e) B.G. Cai, J. Xuan, W.J. Xiao, Sci. Bull. 64 (2019) 337-350;
(f) Y.L. Zhang, K. Sun, Q.Y. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361-1368;
(g) C.G. Feng, M. Ye, K.J. Xiao, S. Li, J.Q. Yu, J. Am. Chem. Soc. 135 (2013) 9322-9325;
(h) Y.M. Li, M. Sun, H.L. Wang, Q.P. Tian, S.D. Yang, Angew. Chem. Int. Ed. 52 (2013) 3972-3976;
(i) L.L. Liao, Y.Y. Gui, X.B. Zhang, D.G. Yu, Org. Lett. 19 (2017) 3735-3738;
(j) Y.H. Li, Y.Y. Zhu, S.D. Yang, Org. Chem. Front. 5 (2018) 822-826;
(k) C. Li, Z.C. Qi, Q. Yang, X.Y. Qiang, S.D. Yang, Chin. J. Chem. 36 (2018) 1052-1058;
(l) R. Isshik, K. Muto, J. Yamaguchi, Org. Lett. 20 (2018) 1150-1153;
(m) J. Yang, T.Q. Chen, L.B. Han, J. Am. Chem. Soc. 137 (2015) 1782-1785;
(n) D.P. Hari, B. König, Org. Lett. 13 (2011) 3852-3855;
(o) Y.L. Zhao, G.J. Wu, F.S. Han, Chem. Commun. 48 (2012) 5868-5870;
(p) V. Qunit, F. Morlet-Savary, J.F. Lohier, et al., J. Am. Chem. Soc. 138 (2016) 7436-7441;
(q) L.B. Niu, J.M. Liu, H. Yi, et al., ACS Catal. 7 (2017) 7412-7416;
(r) L.B. Niu, H. Yi, S.C. Wang, et al., Chem. Commun. 54 (2018) 1659-1662;
(s) C.H. Wang, Y.H. Li, S.D. Yang, Org. Lett. 20 (2018) 2382-2385;
(t) Y.Y. Song, L.L. Wang, Z. Duan, F. Mathey, Chin. Chem. Lett. 31 (2020) 329-332;
(u) Y.N. Ma, S.X. Li, S.D. Yang, Acc. Chem. Res. 50 (2017) 1480-1492.
-
[4]
X.Q. Chu, Y. Zi, H. Meng, X.P. Xu, S.J. Ji, Chem. Commun. 50 (2014) 7642-7645.
doi: 10.1039/c4cc02114b
-
[5]
X. Mi, C. Wang, M. Huang, Y. Wu, Y.J. Wu, Org. Biomol. Chem. 12 (2014) 8394-8397.
doi: 10.1039/C4OB01739K
-
[6]
Y. Yin, W.Z. Weng, J.G. Sun, B. Zhang, Org. Biomol. Chem.16 (2018) 2356-2361.
doi: 10.1039/C8OB00231B
-
[7]
X.Y. Yu, J.R. Chen, W.J. Xiao, et al., Angew. Chem. Int. Ed. 57 (2018) 738-743.
doi: 10.1002/anie.201710618
-
[8]
(a) J.M. Fan, C.F. Wan, Wang Q, et al., Org. Biomol. Chem. 7 (2009) 3168-3172;
(b) F.F. Mo, L.J. Trzepkowski, G.B. Dong, Angew. Chem. Int. Ed. 51 (2012) 13075-13079;
(c) Z. Zhang, C. Li, S.H. Wang, et al., Org. Biomol. Chem. 15 (2017) 3239-3247.
-
[9]
(a) J. Desroches, P.A. Champangne, Y. Behassine, J.K. Paguin, Org. Biomol. Chem. 13 (2015) 2243-2246;
(b) T.W. Bevan, J.F. Taylor, H. Wong, D.T. Northcote, J.E. Harvey, Tetrahedron 74 (2018) 2942-2955.
-
[10]
(a) E.B. Jane, R.S. Davidson, Makromol. Chem. Rapid. Commun. 8 (1987) 311-314;
(b) A.P. William, T.G. Jen, D.F. Church, J. Org. Chem. 50 (1985) 185-189.

 Login In
Login In






 DownLoad:
DownLoad: