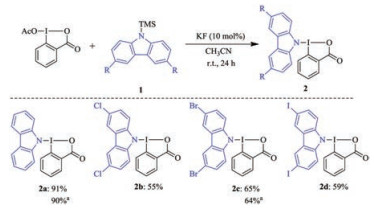
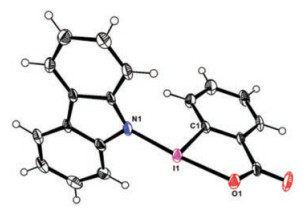
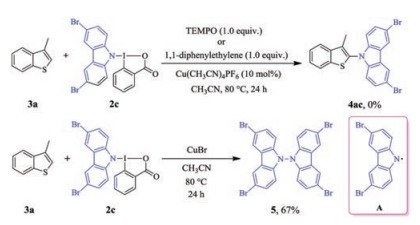
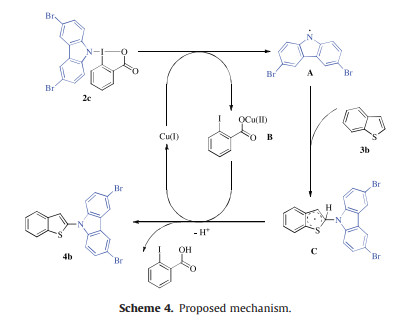
-
[1]
(a) A.W. Schmidt, K.R. Reddy, H.J. Knolker, Chem. Rev. 112 (2012) 3193-3328;
(b) H.J. Knölker, K.R. Reddy, Chem. Rev. 102 (2012) 4303-4427;
(c) Z. Sadiq, E.A. Hussain, S. Naz, Mini-Rev. Org. Chem. 14 (2017) 469-488;
(d) J.F. Morin, M. Leclerc, D. Adès, A. Siove, Macromol. Rapid Commun. 26 (2005) 761-778;
(e) J.V. Grazulevicius, P. Strohriegl, J. Pielichowski, K. Pielichowski, Prog. Polym. Sci. 28 (2003) 1297-1353.
-
[2]
(a) P. Rajakumar, K. Sekar, V. Shanmugaiah, et al., Eur. J. Med. Chem. 44 (2009) 3040-3045;
(b) Z. Liu, R.C. Larock, Tetrahedron 63 (2007) 347-355;
(c) C. Ito, S. Katsuno, M. Itoigawa, et al., J. Nat. Prod. 63 (2000) 125-128.
-
[3]
(a) M.P. Gaj, C. Fuentes-Hernandez, Y. Zhang, S.R. Marder, B. Kippelen, Org. Electron. 16 (2015) 109-112;
(b) Z.M. Hudson, Z. Wang, M.G. Helander, Z.H. Lu, S. Wang, Adv. Mater. 24 (2012) 2922-2928;
(c) L.S. Sapochak, A.B. Padmaperuma, X. Cai, et al., J. Phys. Chem. C 112 (2008) 7989-7996;
(d) M. Baibarac, M. Lira-Cantú, J. Oró Sol, et al., Compos. Sci. Technol. 67 (2007) 2556-2563.
-
[4]
(a) I. Tanaka, Y. Tabata, S. Tokito, Chem. Phys. Lett. 400 (2004) 86-89;
(b) T. Thoms, S. Okada, J.P. Chen, M. Furugori, Thin Solid Films 436 (2003) 264-268.
-
[5]
(a) M.M. Heravi, Z. Kheilkordi, V. Zadsirjan, M. Heydari, M. Malmir, J. Organoment. Chem. 861 (2018) 17-104;
(b) P. Ruiz-Castillo, S.L. Buchwald, Chem. Rev. 116 (2016) 12564-12649;
(c) J. Bariwal, E. Van der Eycken, Chem. Soc. Rev. 42 (2013) 9283-9303.
-
[6]
(a) D.P. Hari, P. Caramenti, J. Waser, Acc. Chem. Res. 51 (2018) 3212-3225;
(b) A. Yoshimura, V.V. Zhdankin, Chem. Rev. 116 (2016) 3328-3435;
(c) Y. Li, D.P. Hari, M.V. Vita, J. Waser, Angew. Chem. Int. Ed. 55 (2016) 4436-4454;
(d) J. Waser, Synlett. 27 (2016) 2761-2773;
(e) J.P. Brand, D.F. González, S. Nicolai, J. Waser, Chem. Commun. (Camb.) 47 (2011) 102-115;
(f) V.V. Zhdankin, Curr. Org. Synth. 2 (2005) 121-145.
-
[7]
(a) V.V. Zhdankin, A.P. Krasutsky, C.J. Kuehl, et al., J. Am. Chem. Soc. 118 (1996) 5192-5197;
(b) M.V. Vita, J. Waser, Org. Lett. 15 (2013) 3246-3249;
(c) A. Sharma, J.F. Hartwig, Nature 517 (2015) 600-604.
-
[8]
(a) V.V. Zhdankin, M. McSherry, B. Mismash, et al., Tetrahedron Lett. 38 (1997) 21-24;
(b) X.H. Hu, X.F. Yang, T.P. Loh, ACS Catal. 6 (2016) 5930-5934.
-
[9]
K. Kiyokawa, T. Kosaka, T. Kojima, S. Minakata, Angew. Chem. Int. Ed. 54 (2015) 13719-13723.
doi: 10.1002/anie.201506805
-
[10]
(a) H. Wang, D. Zhang, H. Sheng, C. Bolm, J. Org. Chem. 82 (2017) 11854-11858;
(b) H. Wang, D. Zhang, H. Sheng, C. Bolm, Chem. -Eur. J. 24 (2018) 14942-14945.
-
[11]
(a) P. Caramenti, S. Nicolai, J. Waser, Chem. -Eur. J. 23 (2017) 14702-14706;
(b) P. Caramenti, R.K. Nandi, J. Waser, Chem. -Eur. J. 24 (2018) 10049-10053.
-
[12]
(a) S. Cai, C. Chen, Z. Sun, C. Xi, Chem. Commun. (Camb.) 49 (2013) 4552-4554;
(b) J. Xie, X. Yuan, A. Abdukader, C. Zhu, J. Ma, Org. Lett. 16 (2014) 1768-1771;
(c) Y. Kuninobu, M. Nishi, M. Kanai, Org. Biomol. Chem. 14 (2016) 8092-8100;
(d) X. Gao, Y. Geng, S. Han, et al., Org. Lett. 20 (2018) 3732-3735.

 Login In
Login In






 DownLoad:
DownLoad: