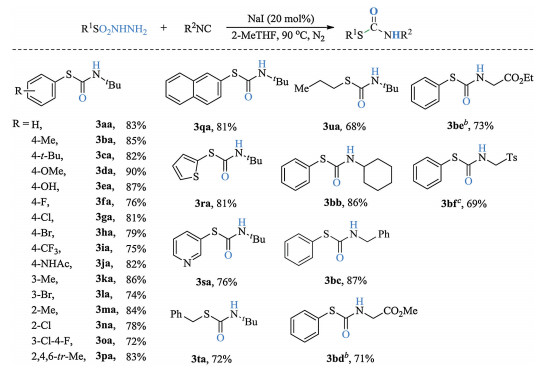
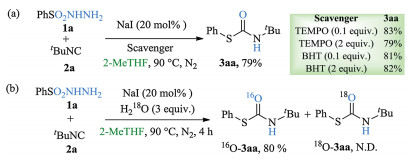
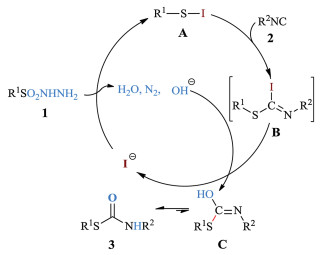
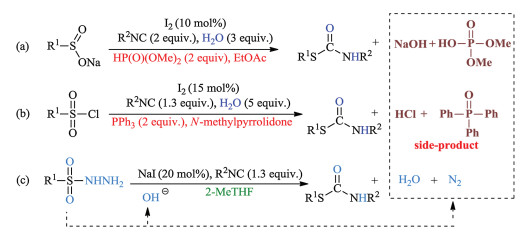
-
[1]
Y. Gu, F. Jerome, Chem. Soc. Rev. 42 (2013) 9550-9570.
doi: 10.1039/c3cs60241a
-
[2]
(a) J. Yang, J.N. Tan, Y. Gu, Green Chem. 14 (2012) 3304-3317;
(b) R. Bai, P. Liu, J. Yang, C. Liu, Y. Gu, ACS Sustainable Chem. Eng. 3 (2015) 1292-1297;
(c) S.G. Zhang, Z.B. Xie, L.S. Liu, M. Liang, Z.G. Le, Chin. Chem. Lett. 28 (2017) 101-104;
(d) G. Gao, P. Wang, P. Liu, et al., Chin. J. Org. Chem. 38 (2018) 846-854;
(e) L. Li, Q. Chen, X. Xiong, et al., Chin. Chem. Lett. 29 (2018) 1893-1896.
-
[3]
(a) H. Li, C. Liu, Y. Zhang, et al., Org. Lett. 17 (2015) 932-935;
(b) D. Cao, Y. Zhang, C. Liu, et al., Org. Lett. 18 (2016) 2000-2003;
(c) B. Lai, R. Bai, Y. Gu, ACS Sustainable Chem. Eng. 6 (2018) 17076-17086;
(d) G.P. Yang, X. Wu, B. Yu, C. Hu, ACS Sustainable Chem. Eng. 7 (2019) 3727-3732;
(e) D.Q. Dong, W.J. Chen, Y. Yang, X. Gao, Z.L. Wang, Chem. Select 4 (2019) 2480-2483.
-
[4]
(a) R. Mohebat, A. Yazdani-Elah-Abadi, M. Malek-Taher, N. Hazeri, Chin. Chem. Lett. 28 (2017) 943-948;
(b) L.Y. Xie, Y. Duan, L.H. Lu, et al., ACS Sustainable Chem. Eng. 5 (2017) 10407-10412;
(c) Q.Q. Xuan, Y.H. Wei, Q.L. Song, Chin. Chem. Lett. 28 (2017) 1163-1166;
(d) D.Q. Dong, S.H. Hao, H. Zhang, Z.L. Wang, Chin. Chem. Lett. 28 (2017) 1597-1599;
(e) M. Zhang, Q.Y. Fu, G. Gao, et al., ACS Sustainable Chem. Eng. 5 (2017) 6175-6182;
(f) H. Cui, W. Wei, D. Yang, et al., Green Chem. 19 (2017) 3520-3524;
(g) K. Sun, X.L. Chen, S.J. Li, et al., J. Org. Chem. 83 (2018) 14419-14430;
(h) P. Bao, L. Wang, Q. Liu, et al., Tetrahedron Lett. 60 (2019) 214-218;
(i) Y. Liu, L. Chen, Z. Wang, et al., J. Org. Chem. 84 (2019) 204-215;
(j) Y. Zheng, M. Liu, G. Qiu, W. Xie, J. Wu, Tetrahedron 75 (2019) 1663-1668;
(k) Y.H. Wang, B. Ouyang, G. Qiu, H. Zhou, J.B. Liu, Org. Biomol. Chem. 17 (2019) 4335-4341;
(l) K. Sun, S.J. Li, X.L. Chen, et al., Chem. Commun. 55 (2019) 2861-2864;
(m) J. Li, W. Tang, D. Ren, J. Xu, Z. Yang, Green Chem. 21 (2019) 2088-2094;
(n) Y.C. Wang, R.X. Wang, G. Qiu, et al., Org. Chem. Front. 6 (2019) 2471-2479.
-
[5]
A.D. Mamuye, S. Monticelli, L. Castoldi, W. Holzer, V. Pace, Green Chem. 17 (2015) 4194-4197.
doi: 10.1039/C5GC01002K
-
[6]
(a) W. Xie, S. Xie, Y. Zhou, et al., Eur. J. Med. Chem. 81 (2014) 22-27;
(b) X. Gong, J. Chen, X. Li, W. Xie, J. Wu, Chem. -Asian J. 13 (2018) 2543-2548;
(c) W. Xie, Y. Wu, J. Zhang, et al., Eur. J. Med. Chem. 145 (2018) 35-40;
(d) L. Xue, Y. Liu, W. Qin, H. Yan, Chin. Chem. Lett. 29 (2018) 1215-1218;
(e) Q. Liang, Y. Zhang, M. Zeng, et al., Toxicol. Res. 7 (2018) 521-528;
(f) G. Xiao, H. Min, Z. Zheng, G. Deng, Y. Liang, Chin. Chem. Lett. 29 (2018) 1363-1366;
(g) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1603;
(h) H. Jiang, X. Tang, Z. Xu, et al., Org. Biomol. Chem. 17 (2019) 2715-2720;
(i) J. Xu, W. Huang, R. Bai, et al., Green Chem. 21 (2019) 2061-2069;
(j) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. (2019), doi: http://dx.doi.org/10.1016/j.cclet.2019.05.041;
(k) D.Q. Dong, L.X. Li, G.H. Li, Q. Deng, Z.L. Wang, Chin. J. Catal. 40 (2019) 1494-1498;
(l) Y. Liu, X.L. Chen, K. Sun, et al., Org. Lett. 21 (2019) 4019-4024;
(m) A. El-Harairy, Y. Qi, B. Lai, et al., Adv. Synth. Catal. 361 (2019) 3342-3350;
(n) C. Zhu, D. Wei, Y. Wu, et al., J. Alloys Compd. 778 (2019) 731-740;
(o) F.L. Zeng, X.L. Chen, S.Q. He, et al., Org. Chem. Front. 6 (2019) 1476-1480;
(p) X.M. Xu, D.M. Chen, Z.L. Wang, Chin. Chem. Lett. (2019), doi: http://dx.doi.org/10.1016/j.cclet.2019.05.048;
(q) X. Gong, G. Li, Z. Gan, et al., Asian J. Org. Chem. 8 (2019) 1472-1478.
-
[7]
(a) T. Mizuno, I. Nishiguchi, T. Okushi, T. Hirashima, Tetrahedron Lett. 32 (1991) 6867-6868;
(b) A.W. Erian, S.M. Sherif, Tetrahedron 55 (1999) 7957-8024;
(c) J. Zhu, B. Xu, J. Yu, et al., Org. Biomol. Chem. 16 (2018) 5999-6005.
-
[8]
(a) R.S. Pathare, V. Patil, H. Kaur, et al., Org. Biomol. Chem. 16 (2018) 8263-8266;
(b) W.H. Bao, C. Wu, J.T. Wang, et al., Org. Biomol. Chem. 16 (2018) 8403-8407;
(c) W. Wei, P. Bao, H. Yue, et al., Org. Lett. 20 (2018) 5291-5295;
(d) X. Gong, X. Li, W. Xie, J. Wu, S. Ye, Org. Chem. Front. 6 (2019) 1863-1867.
-
[9]
(a) G. Li, Z. Gan, K. Kong, X. Dou, D. Yang, Adv. Synth. Catal. 361 (2019) 1808-1814;
(b) Y. Zong, Y. Lang, M. Yang, et al., Org. Lett. 21 (2019) 1935-1938;
(c) X.Q. Chu, D. Ge, T.P. Loh, Z.L. Shen, Org. Chem. Front. 6 (2019) 835-840;
(d) X. Wang, M. Yang, W. Xie, X. Fan, J. Wu, Chem. Commun. 55 (2019) 6010-6013;
(e) G.H. Li, D.Q. Dong, Q. Deng, S.Q. Yan, Z.L. Wang, Synthesis 51 (2019) 3313-3319;
(f) F.S. He, Y. Wu, X. Li, H. Xia, J. Wu, Org. Chem. Front. 6 (2019) 1873-1878;
(g) K. Sun, Z. Shi, Z. Liu, et al., Org. Lett. 20 (2018) 6687-6690;
(h) J. Zhang, X. Li, W. Xie, S. Ye, J. Wu, Org. Lett. 21 (2019) 4950-4954;
(i) S. Ye, T. Xiang, X. Li, J. Wu, Org. Chem. Front. 6 (2019) 2183-2199.
-
[10]
P. Bao, L. Wang, H. Yue, et al., J. Org. Chem. 84 (2019) 2976-2983.
doi: 10.1021/acs.joc.8b02844
-
[11]
W.H. Bao, M. He, J.T. Wang, et al., J. Org. Chem. 84 (2019) 6065-6071.
doi: 10.1021/acs.joc.9b00178
-
[12]
(a) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. China Chem. 62 (2019) 460-464;
(b) Y. You, K. Zhou, B. Guo, et al., ACS Sens. 4 (2019) 774-779;
(c) T.Y. Shang, L.H. Lu, Z. Cao, et al., Chem. Commun. 55 (2019) 5408-5419;
(d) L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240;
(e) L. Peng, Z. Hu, Q. Lu, et al., Chin. Chem. Lett. (2019), doi: http://dx.doi.org/10.1016/j.cclet.2019.05.063;
(f) Q. Zhu, C. Liu, L. Zhou, et al., Biosens. Bioelectron. 140 (2019) 111356;
(g) K.J. Liu, Z.H. Duan, X.L. Zeng, et al., ACS Sustainable Chem. Eng. 7 (2019) 10293-10298;
(h) L.Y. Xie, T.G. Fang, J.X.T. Tan, et al., Green Chem. 21 (2019) 3858-3863;
(i) L.Y. Xie, L.L. Jiang, J.X. Tan, et al., ACS Sustainable Chem. Eng. 7 (2019) 14153-14160.
-
[13]
(a) W. Zhao, P. Xie, Z. Bian, et al., J. Org. Chem. 80 (2015) 9167-9175;
(b) X. Pang, L. Xiang, X. Yang, R. Yan, Adv. Synth. Catal. 358 (2016) 321-325.

 Login In
Login In






 DownLoad:
DownLoad: