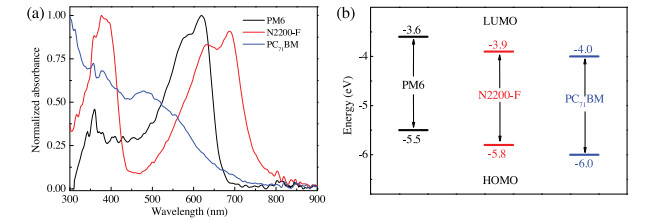
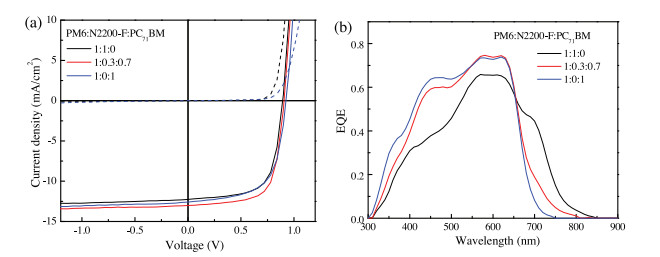
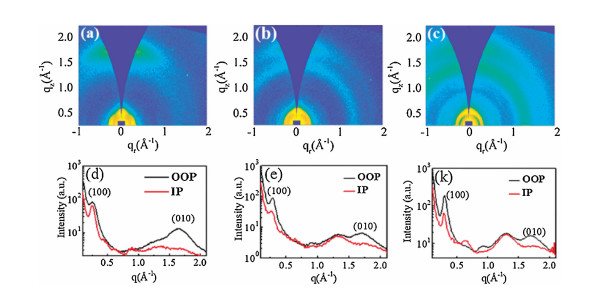
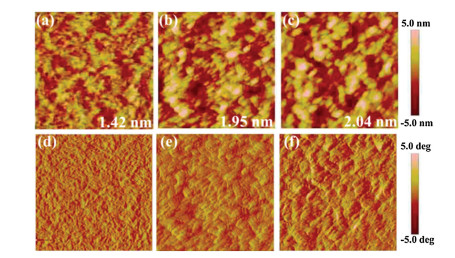
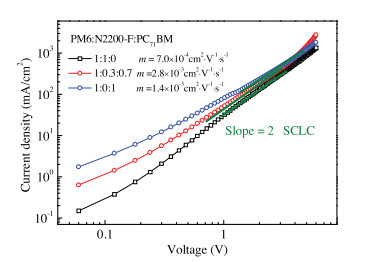
-
[1]
G. Yu, J. Gao, J.C. Hummelen, F. Wudl, A.J. Heeger, Science 270(1995) 1789-1791.
doi: 10.1126/science.270.5243.1789
-
[2]
(a) J.K. Lee, W.L. Ma, A.J. Heeger, et al., J. Am. Chem. Soc.130(2008) 3619-3623;
(b) J.B. Zhao, W. Ma, H. Yan, et al., Nat. Energy 1(2016) 15027;
(c) L.T. Dou, Y.S. Liu, Z.R. Hong, G. Li, Y. Yang, Chem. Rev. 115(2015) 12633-12665;
(d) Y.F. Li, Acc. Chem. Res. 45(2012) 723-733;
(e) L.Y. Lu, T.Y. Zheng, L.P. Yu, et al., Chem. Rev. 115(2015) 12666-12731;
(f) H.F. Yao, L. Ye, J.H. Hou, et al., Chem. Rev. 116(2016) 7397-7457;
(g) J.S. Song, Z.S. Bo, Sci. China Chem. 62(2019) 9-13.
-
[3]
G.Y. Zhang, J.B. Zhao, H. Yan, et al., Chem. Rev. 118(2018) 3447-3507.
doi: 10.1021/acs.chemrev.7b00535
-
[4]
Y.Z. Lin, J.Y. Wang, X.W. Zhan, et al., Adv. Mater. 27(2015) 1170-1174.
doi: 10.1002/adma.201404317
-
[5]
(a) C.Q. Yan, S. Barlow, X.W. Zhan, et al., Nat. Rev. Mater. 3(2018) 18003;
(b) J.Q. Zhang, H.S. Tan, X.G. Guo, A. Facchetti, H. Yan, Nat. Energy 3(2018) 720-731;
(c) J. Yuan, Y.Q. Zhang, Y.P. Zou, et al., Joule 3(2019) 1140-1151.
-
[6]
(a) Y. Cui, H.F. Yao, J.Q. Zhang, et al., Nat. Commun. 10(2019) 2515;
(b) X.P. Xu, W. Ma, Q. Peng, et al., Adv. Mater. (2019) 1901872.
-
[7]
(a) Q.S. An, F.J. Zhang, B. Hu, et al., Energy Environ. Sci. 9(2016) 281-322;
(b) Z.J. Li, W. Zhang, Q. Peng, et al., J. Mater. Chem. C: Mater. Opt. Electron. Devices 6(2018) 9119-9129;
(c) X.P. Xu, W. Ma, Q. Peng, et al., Adv. Mater. 29(2017) 1704271;
(d) C.P. Chen, Y.Y. Tsai, Y.C. Chen, Y.H. Li, Sol. Energy 176(2018) 170-177.
-
[8]
(a) J.Q. Mai, T.K. Lau, X.H. Lu, et al., Chem. Mater. 28(2016) 6186-6195;
(b) L. Zhong, H.J. Bin, Y.F. Li, et al., J. Mater. Chem. A Mater. Energy Sustain. 6(2018) 24814-24822.
-
[9]
(a) T. Zhang, X.L. Zhao, D.L. Yang, Y.M. Tian, X.N. Yang, Adv. Energy Mater. 8(2018) 1701691;
(b) Z.C. Zhou, F. Liu, X.Z. Zhu, et al., Nat. Energy 3(2018) 952-959.
-
[10]
(a) Y.T. Guo, A.D. Zhang, C. Li, W.W. Li, D.B. Zhu, Chin. Chem. Lett. 29(2018) 371-373;
(b) C.S. Yu, C. Li, W.W. Li, et al., Chin. Chem. Lett. 29(2018) 325-327;
(c) C.S. Yu, J.Y. Li, W.W. Li, Chin. J. Chem. 36(2018) 515-518;
(d) S.C. Zhou, Y.G. Wu, W.W. Li, et al., Acta Phys. Chim. Sin. 34(2018) 344-347;
(e) Y. Li, Y.H. Xu, W.W. Li, et al., Chin. Chem. Lett. 30(2019) 222-224;
(f) F. Yang, C. Li, W.W. Li, et al., Chin. J. Polym. Sci. 35(2017) 239-248.
-
[11]
(a) W.B. Lai, C. Li, W.W. Li, et al., Chem. Mater. 29(2017) 7073-7077;
(b) G.T. Feng, J.Y. Li, W.W. Li, et al., J. Am. Chem. Soc.139(2017) 18647-18656;
(c) G.T. Feng, J.Y. Li, Y.K. He, et al., Joule 3(2019) 1765-1781.
-
[12]
(a) S.Q. Zhang, Y.P. Qin, J. Zhu, J.H. Hou, Adv. Mater. 30(2018) 1800868;
(b) J.W. Jung, J.W. Jo, A.K.Y. Jen, et al., Adv. Mater. 27(2015) 3310-3317.

 Login In
Login In

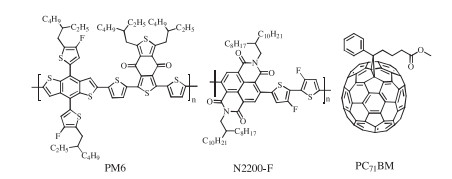





 DownLoad:
DownLoad: