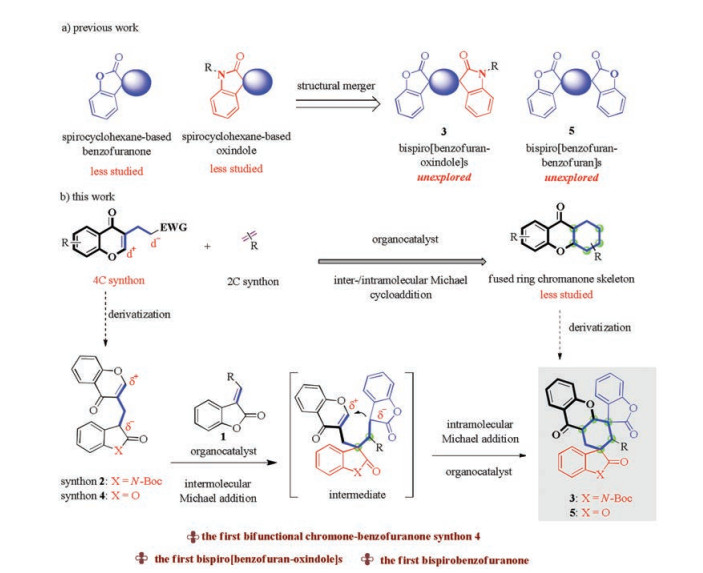
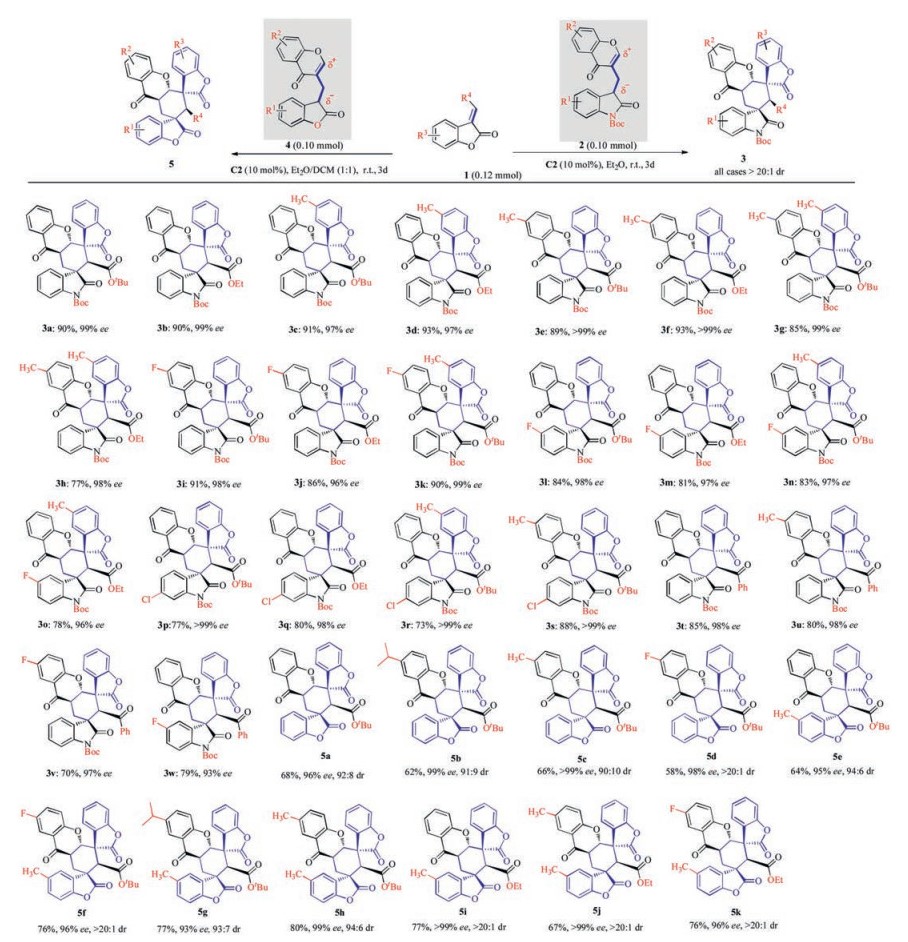
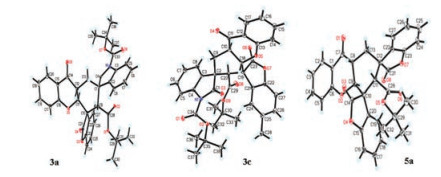
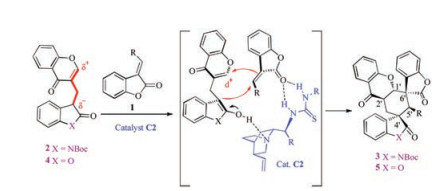
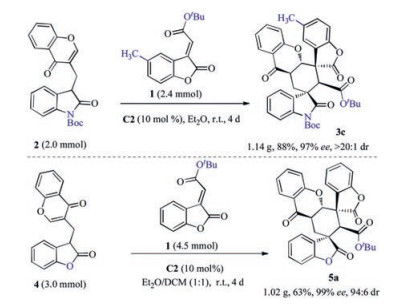
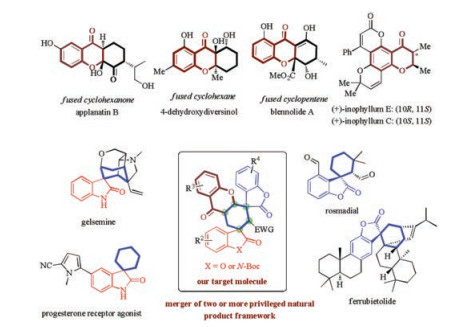
-
[1]
(a) C. Viegas-Junior, A. Danuello, V.D.S. Bolzani, E.J. Barreiro, C.A.M. Fraga, Curr. Med. Chem. 14 (2007) 1829-1852;
(b) S. Fortin, G. Bérubé, Expert Opin. Drug Discov. 8 (2013) 1029-1047;
(c) E.J. Sorensen, H.M.L. Davies, Chem. Soc. Rev. 38 (2009) 2969-2969;
(d) C. Ma, F. Jiang, F.T. Sheng, et al., Angew. Chem. Int. Ed. 58 (2019) 3014-3020;
(e) X.P. Zeng, J. Zhou, J. Am. Chem. Soc. 138 (2016) 8730-8733.
-
[2]
(a) D.S. Tan, Nat. Chem. Biol. 1 (2005) 74-84;
(b) M.D. Burke, S.L. Schreiber, Angew. Chem. Int. Ed. 43 (2004) 46-58;
(c) C. Ma, J.Y. Zhou, Y.Z. Zhang, et al., Chem. -Asian J. 13 (2018) 2549-2558;
(d) M. Sun, C. Ma, S.J. Zhou, et al., Angew. Chem. Int. Ed. 58 (2019) 8703-8708.
-
[3]
(a) A.A. Hussein, J.J.M. Meyer, M.L. Jimeno, B. Rodríguez, J. Nat. Prod. 70 (2007) 293-295;
(b) H.M. Ge, C.H. Zhu, D.H. Shi, et al., Chem. -Eur. J. 14 (2008) 376-381;
(c) L. Pérez-Fons, M.T. Garzón, V. Micol, J. Agric. Food Chem. 58 (2010) 161-171;
(d) M.W. Pertino, C. Theoduloz, J.A. Rodríguez, V. Lazo, J. Nat. Prod. 73 (2010) 639-643;
(e) K.C. Nicolaou, T.R. Wu, Q. Kang, D.Y.K. Chen, Angew. Chem. Int. Ed. 48 (2009) 3440-3443;
(f) K.C. Nicolaou, Q. Kang, T.R. Wu, C.S. Lim, D.Y.K. Chen, J. Am. Chem. Soc. 132 (2010) 7540-7548;
(g) B.Sontag, M.Ruth, P.Spiteller, etal., Eur.J.Org.Chem.2006 (2006)1023-1033;
(h) N. Nakatani, R. Inatani, Agric. Biol. Chem. 47 (1983) 353-358.
-
[4]
(a) X. Li, C. Yang, J.L. Jin, X.S. Xue, J.P. Cheng, Chem. -Asian J. 8 (2013) 997-1003;
(b) C. Cassani, X. Tian, E.C. Escudero-Adán, P. Melchiorre, Chem. Commun. 47 (2011) 233-235.
-
[5]
(a) X. Li, M.H. Lin, Y. Han, F. Wang, J.P. Cheng, Org. Lett. 16 (2014) 114-117;
(b) K. Albertshofer, B. Tan, C.F. Barbas III, Org. Lett. 15 (2013) 2958-2961;
(c) X. Companyó, A. Zea, A.N.R. Alba, et al., Chem. Commun. 46 (2010) 6953-6955.
-
[6]
(a) D. Cheng, Y. Ishihara, B. Tan, C.F. Barbas III, ACS Catal. 4 (2014) 743-762;
(b) G.S. Singh, Z.Y. Desta, Chem. Rev. 112 (2012) 6104-6155;
(c) L. Hong, R. Wang, Adv. Synth. Catal. 355 (2013) 1023-1053;
(d) W.Y. Han, J.Q. Zhao, J. Zuo, et al., Adv. Synth. Catal. 357 (2015) 3007-3031;
(e) J.S. Yu, F. Zhou, Y.L. Liu, J. Zhou, Synlett 26 (2015) 2491-2504;
(f) F. Zhou, Y.L. Liu, J. Zhou, Adv. Synth. Catal. 352 (2010) 1381-1407;
(g) L. Hong, R. Wang, Adv. Synth. Catal. 355 (2013) 1023-1053;
(h) G. Mei, F. Shi, Chem. Commun. 54 (2018) 6607-6621;
(i) Z.Y. Cao, F. Zhou, J. Zhou, Acc. Chem. Res. 51 (2018) 1443-1454;
(j) Y. Zhu, J. Zhou, S. Jin, et al., Chem. Commun. 53 (2017) 11201-11204;
(k) J. Guo, X. Bai, Q. Wang, Z. Bu, J. Org. Chem. 83 (2018) 3679-3687;
(l) Y. Chen, B.D. Cui, Y. Wang, et al., J. Org. Chem. 83 (2018) 10465-10475;
(m) H. Lu, X. Li, F. Shi, S.J. Tu, Chem. -Eur. J. 20 (2014) 11382-11389;
(n) G.J. Mei, D. Li, G.X. Zhou, et al., Chem. Commun. 53 (2017) 10030-10033;
(o) Y. Zhu, J. Zhou, S. Jin, et al., Chem. Commun. 53 (2017) 11201-11204.
-
[7]
(a) F. Wong, H. Watson, A. Gerbes, et al., Gut 61 (2012) 108;
(b) P. Ginès, F. Wong, H. Watson, et al., Hepatology 48 (2008) 204-213;
(c) G.C. Bignan, K. Battista, P.J. Connolly, et al., Bioorg. Med. Chem. Lett. 15 (2005) 5022-5026.
-
[8]
(a) Y.K. Liu, M. Nappi, E. Arceo, S. Vera, P. Melchiorre, J. Am. Chem. Soc. 133 (2011) 15212-15218;
(b) I. Chatterjee, D. Bastida, P. Melchiorre, Adv. Synth. Catal. 355 (2013) 3124-3130;
(c) B. Zhou, Y. Yang, J. Shi, Z. Luo, Y. Li, J. Org. Chem. 78 (2013) 2897-2907;
(d) K. Jiang, Z.J. Jia, S. Chen, L. Wu, Y.C. Chen, Chem. -Eur. J. 16 (2010) 2852-2856.
-
[9]
(a) J. Chen, J.M. Chen, F. Lang, et al., J. Am. Chem. Soc. 132 (2010) 4552-4553;
(b) M.K. Brown, S.J. Degrado, A.H. Hoveyda, Angew. Chem. Int. Ed. 44 (2005) 5306-5310;
(c) D. Xiong, W. Zhou, Z. Lu, S. Zeng, J. Wang, Chem. Commun. 53 (2017) 6844-6847;
(d) L. Meng, M.Y. Jin, J. Wang, Org. Lett. 18 (2016) 4986-4989;
(e) B.M. Trost, E. Gnanamani, C.A. Kalnmals, C. Hung, J.S. Tracy, J. Am. Chem. Soc. 141 (2019) 1489-1493.
-
[10]
(a) G. Wen, Y. Su, G. Zhang, et al., Org. Lett. 18 (2016) 3980-3983;
(b) A. Khilevich, A. Mar, M.T. Flavin, et al., Tetrahedron Asymmetry 7 (1996) 3315-3326;
(c) E. Sekino, T. Kumamoto, T. Tanaka, T. Ikeda, T. Ishikawa, J. Org. Chem. 69 (2004) 2760-2767;
(d) J. Garcia, S. Barluenga, K. Beebe, L. Neckers, N. Winssinger, Chem. -Eur. J. 16 (2010) 9767-9771;
(e) M.M. Biddle, M. Lin, K.A. Scheidt, J. Am. Chem. Soc. 129 (2007) 3830-3831;
(f) R.L. Farmer, K.A. Scheidt, Chem. Sci. 4 (2013) 3304-3309;
(g) B.R. McDonald, A.E. Nibbs, K.A. Scheidt, Org. Lett. 17 (2015) 98-101.
-
[11]
(a) K.S. Masters, S. Bräse, Chem. Rev. 112 (2012) 3717-3776;
(b) R.N. Kharwar, A. Mishra, S. Gond, K.A. Stierle, D. Stierle, Nat. Prod. Rep. 28 (2011) 1208-1228;
(c) S. Sato, Y. Suga, T. Yoshimura, et al., Bioorg. Med. Chem. Lett. 9 (1999) 2653-2656;
(d) S.H. Shim, J. Baltrusaitis, J.B. Gloer, D.T. Wicklow, J. Nat. Prod. 74 (2011) 395-401;
(e) R. Goel, V. Sharma, A. Budhiraja, M.P.S. Ishar, Bioorg. Med. Chem. Lett. 22 (2012) 4665-4667;
(f) F. Zhang, L. Li, S. Niu, et al., J. Nat. Prod. 75 (2012) 230-237;
(g) P.S. Wang, P. Liu, Y.J. Zhai, et al., J. Am. Chem. Soc. 137 (2015) 12732-12735;
(h) M.C. Brohmer, E. Bourcet, M. Nieger, S. Brase, Chem. -Eur. J. 17 (2011) 13706-13711;
(i) K.C. Nicolaou, A. Li, Angew. Chem. Int. Ed. 47 (2008) 6579-6582;
(j) J.L. Li, S.L. Zhou, P.Q. Chen, et al., Chem. Sci. 3 (2012) 1879-1882.
-
[12]
(a) Ł. Albrecht, F. CruzAcosta, A. Fraile, et al., Angew. Chem. Int. Ed. 51 (2012) 9088-9092;
(b) A. Albrecht, J. Bojanowski, Adv. Synth. Catal. 359 (2017) 2907-2911;
(c) A. Danda, N. Kesava-Reddy, C. Golz, C. Strohmann, K. Kumar, Org. Lett. 18 (2016) 2632-2635.
-
[13]
(a) X.L. Liu, G. Zhou, Y. Gong, et al., Org. Lett. 21 (2019) 2528-2531;
(b) X.L. Liu, Y. Gong, S. Chen, et al., Org. Chem. Front. 6 (2019) 1603-1607.

 Login In
Login In






 DownLoad:
DownLoad: