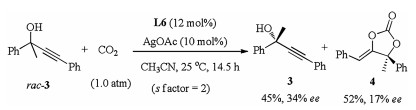
-
[1]
(a) T. Sakakura, J.C. Choi, H. Yasuda, Chem. Rev. 107 (2007) 2365-2387;
(b) D.M. D'Alessandro, B. Smit, J.R. Long, Angew. Chem. Int. Ed. 49 (2010) 6058-6082;
(c) M. Cokoja, C. Bruckmeier, B. Rieger, W.A. Herrmann, F.E. Kühn, Angew. Chem. Int. Ed. 50 (2011) 8510-8537;
(d) E.V. Kondratenko, G. Mul, J. Baltrusaitis, G.O. Larraźabalc, J. Pérez-Ramírez, Energy Environ. Sci. 6 (2013) 3112-3135;
(e) M. Aresta, A. Dibenedetto, A. Angelini, Chem. Rev. 114 (2014) 1709-1742;
(f) X.B. Lu, Carbon Dioxide and Organometallics, Springer, Switzerland, (2016);
(g) A.W. Kleij, M. North, A. Urakawa, ChemSusChem 10 (2017) 1036-1038;
(h) S.S. Yan, Q. Fu, L.L. Liao, et al., Coord. Chem. Rev. 374 (2018) 439-463;
(i) S. Wang, C. Xi, Chem. Soc. Rev. 48 (2019) 382-404.
-
[2]
M. He, Y. Sun, B. Han, Angew. Chem. Int. Ed. 52 (2013) 9620-9633.
doi: 10.1002/anie.201209384
-
[3]
(a) Q. Liu, L. Wu, R. Jackstell, M. Beller, Nat. Commun. 6 (2015) 5933-5947;
(b) C. Maeda, Y. Miyazaki, T. Ema, Catal. Sci. Technol. 4 (2014) 1482-1497;
(c) G. Fiorani, W. Guo, A.W. Kleij, Green Chem. 17 (2015) 1375-1389;
(d) M. Börjesson, T. Moragas, D. Gallego, R. Martin, ACS Catal. 6 (2016) 6739-6749;
(e) S.N. Riduan, Y. Zhang, Dalton Trans. 39 (2010) 3347-3357;
(f) W. Zhang, C. Guo, X. Lü, Chin. J. Catal. 37 (2016) 215-217;
(g) Z. Zhang, T. Ju, J.H. Ye, D.G. Yu, Synlett 28 (2017) 741-750;
(h) W. Zhang, N. Zhang, C. Guo, X. Lü, Chin. J. Org. Chem. 37 (2017) 1309-1321;
(i) Y.Y. Gui, W.J. Zhou, J.H. Ye, D.G. Yu, ChemSusChem 10 (2017) 1337-1340;
(j) Y. Cao, X. He, N. Wang, H.R. Li, L.N. He, Chin. J. Chem. 36 (2018) 644-659;
(k) Z. Zhang, J.H. Ye, D.S. Wu, Y.Q. Zhou, D.G. Yu, Chem. -Asian J. 13 (2018) 2292-2306.
-
[4]
(a) J.W. Comerford, I.D.V. Ingram, M. North, X. Wu, Green Chem. 17 (2015) 1966-1987;
(b) Q.W. Song, L.N. He, Adv. Synth. Catal. 358 (2016) 1251-1258;
(c) B. Zou, Chin. J. Chem. 35 (2017) 541-550;
(d) Y. Wu, Y. Zhao, R. Li, et al., ACS Catal. 7 (2017) 6251-6255;
(e) H. Zhou, G.X. Wang, X.B. Lu, Asian J. Org. Chem. 6 (2017) 1264-1269;
(f) S. Sun, B. Wang, N. Gu, J.T. Yu, J. Cheng, Org. Lett. 19 (2017) 1088-1091;
(g) G. Shen, W.J. Zhou, X.B. Zhang, et al., Chem. Commun. 54 (2018) 5610-5613.
-
[5]
H. Zhang, H.B. Liu, J.M. Yue, Chem. Rev. 114 (2014) 883-898.
doi: 10.1021/cr300430e
-
[6]
(a) J. Vaitla, Y. Guttormsen, J.K. Mannisto, et al., ACS Catal. 7 (2017) 7231-7244;
(b) N. Kielland, C.J. Whiteoak, A.W. Kleija, Adv. Synth. Catal. 355 (2013) 2115-2138;
(c) X.B. Lu, B. Liang, Y.J. Zhang, et al., J. Am. Chem. Soc. 126 (2004) 3732-3733;
(d) M. Takimoto, Y. Nakamura, K. Kimura, M. Mori, J. Am. Chem. Soc. 126 (2004) 5956-5957;
(e) M. Zhang, X. Zhao, S. Zheng, Chem. Commun. 50 (2014) 4455-4458;
(f) B.A. Vara, T.J. Struble, W. Wang, M.C. Dobish, J.N. Johnston, J. Am. Chem. Soc. 137 (2015) 7302-7305;
(g) Y.Y. Gui, N. Hu, X.W. Chen, et al., J. Am. Chem. Soc. 139 (2017) 17011-17014.
-
[7]
(a) M. Yoshida, M. Fujita, T. Ishii, M. Ihara, J. Am. Chem. Soc. 125 (2003) 4874-4881;
(b) S. Yoshida, K. Fukui, S. Kikuchi, T. Yamada, J. Am. Chem. Soc. 132 (2010) 4072-4073.
-
[8]
(a) J.M. Keith, J.F. Larrow, E.N. Jacobsen, Adv. Synth. Catal. 343 (2001) 5-26;
(b) E. Vedejs, M. Jure, Angew. Chem. Int. Ed. 44 (2005) 3974-4001;
(c) H. Pellissier, Adv. Synth. Catal. 353 (2011) 1613-1666;
(d) C.E. Müller, P.R. Schreiner, Angew. Chem. Int. Ed. 50 (2011) 6012-6042;
(e) R. Gurubrahamam, Y.S. Cheng, W.Y. Huang, K. Chen, ChemCatChem 8 (2016) 86-96;
(f) O. Verho, J.E. Backvall, J. Am. Chem. Soc. 137 (2015) 3996-4009;
(g) G. Ma, M.P. Sibi, Chem. -Eur. J. 21 (2015) 11644-11657.
-
[9]
(a) Y. Zhu, L. Sun, P. Lu, Y. Wang, ACS Catal. 4 (2014) 1911-1925;
(b) Q. Wang, L. Pu, Synlett 24 (2013) 1340-1363;
(c) P.J. Stang, F. Diederich, Modern Acetylene Chemistry, Wiley-VCH: Weinheim, 2008.
-
[10]
(a) Q.W. He, S.M. Ma, Chinese J. Org. Chem. 22 (2002) 375-387;
(b) L. Pu, Tetrahedron 59 (2003) 9873-9886;
(c) G. Lu, Y.M. Li, X.S. Li, A.S.C. Chan, Coordin. Chem. Rev. 249 (2005) 1736-1744;
(d) B.M. Trost, A.H. Weiss, Adv. Synth. Catal. 351 (2009) 963-983;
(e) B.M. Trost, M.J. Bartlett, A.H. Weiss, A.J.V. Wangelin, V.S. Chan, Chem. -Eur. J. 18 (2012) 16498-16509;
(f) V. Bisai, V.K. Singh, Tetrahedron Lett. 57 (2016) 4771-4784;
(g) H. Noda, N. Kumagai, M. Shibasaki, Asian J. Org. Chem. 7 (2018) 599-612.
-
[11]
(a) B. Jiang, Z. Chen, X. Tang, Org. Lett. 4 (2002) 3451-3453;
(b) P.G. Cozzi, Angew. Chem. Int. Ed. 42 (2003) 2895-2898;
(c) G. Lu, X. Li, X. Jia, W.L. Chan, A.S.C. Chan, Angew. Chem. Int. Ed. 42 (2003) 5057-5058;
(d) P.G. Cozzi, S. Alesi, Chem. Commun. (2004) 2448-2449;
(e) C. Chen, L. Hong, Z.Q. Xu, L. Liu, R. Wang, Org. Lett. 8 (2006) 2277-2280;
(f) K. Tanaka, K. Kukita, T. Ichibakase, S. Kotanib, M. Nakajima, Chem. Commun. 47 (2011) 5614-5616;
(g) T. Ohshima, T. Kawabata, Y. Takeuchi, et al., Angew. Chem. Int. Ed. 50 (2011) 6296-6300;
(h) T. Wang, J.L. Niu, S.L. Liu, et al., Adv. Synth. Catal. 355 (2013) 927-937;
(i) P. Smirnov, J. Mathew, A. Nijs, et al., Angew. Chem. Int. Ed. 52 (2013) 13717-13721;
(j) Y. Zheng, Y. Tan, K. Harms, et al., J. Am. Chem. Soc. 139 (2017) 4322-4325;
(k) B.P. Zavesky, J.S. Johnson, Angew. Chem. Int. Ed. 56 (2017) 8805-8808;
(l) M.C. Schwarzer, A. Fujioka, T. Ishii, et al., Chem. Sci. 9 (2018) 3484-3493;
(m) D.K. Friel, M.L. Snapper, A.H. Hoveyda, J. Am. Chem. Soc.130 (2008) 9942-9951;
(n) H. Kawai, K. Tachi, E. Tokunaga, M. Shiro, N. Shibata, Org. Lett. 12 (2010) 5104-5107;
(o) H. Noda, F. Amemiya, K. Weidner, N. Kumagai, M. Shibasaki, Chem. Sci. 8 (2017) 3260-3269;
(p) W. Zhang, S. Ma, Chem. Commun. 54 (2018) 6064-6067.
-
[12]
(a) Z.Y. Cao, F. Zhou, J. Zhou, Acc. Chem. Res. 51 (2018) 1443-1454;
(b) Y.L. Liu, X.P. Yin, J. Zhou, Chin. J. Chem. 36 (2018) 321-328;
(c) X.P. Zeng, J. Zhou, J. Am. Chem. Soc. 138 (2016) 8730-8733;
(d) Z.Y. Cao, J.S. Jiang, J. Zhou, Org. Biomol. Chem. 14 (2016) 5500-5504;
(e) X.P. Zeng, Z.Y. Cao, X. Wang, et al., J. Am. Chem. Soc. 138 (2016) 416-425;
(f) Y.L. Liu, F.M. Liao, Y.F. Niu, X.L. Zhao, J. Zhou, Org. Chem. Front.1 (2014) 742-747;
(g) Y.L. Liu, J. Zhou, Acta Chim. Sinica 70 (2012) 1451-1456;
(h) Y.L. Liu, J. Zhou, Chem. Commun. 48 (2012) 1919-1921;
(i) Z.Y. Cao, Y. Zhang, C.B. Ji, J. Zhou, Org. Lett. 13 (2011) 6398-6401;
(j) Y.L. Liu, B.L. Wang, J.J. Cao, et al., J. Am. Chem. Soc.132 (2010) 15176-15178;
(k) J.J. Cao, F. Zhou, J. Zhou, Angew. Chem. Int. Ed. 49 (2010) 4976-4980.
-
[13]
(a) X.T. Gao, C.C. Gan, S.Y. Liu, et al., ACS Catal. 7 (2017) 8588-8593;
(b) F. Zhou, S.L. Xie, X.T. Gao, et al., Green Chem. 19 (2017) 3908-3915.
-
[14]
(a) S. Kotani, K. Kukita, K. Tanaka, et al., J. Org. Chem. 79 (2014) 4817-4825;
(b) B. Bieszczad, D.G. Gilheany, Angew. Chem. Int. Ed. 56 (2017) 4272-4276.

 Login In
Login In






 DownLoad:
DownLoad: