Citation:
Xu Xin-Ming, Chen De-Mao, Wang Zu-Li. Recent advances in sulfenylation of C(sp3)-H bond under transition metal-free conditions[J]. Chinese Chemical Letters,
;2020, 31(1): 49-57.
doi:
10.1016/j.cclet.2019.05.048

-
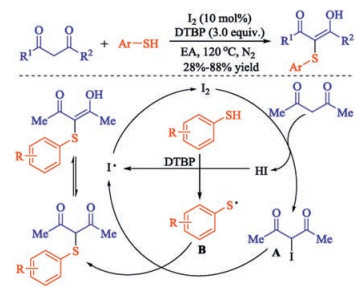
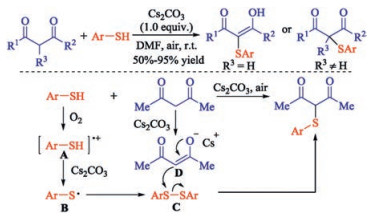
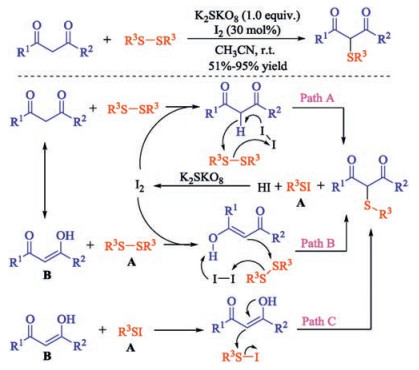
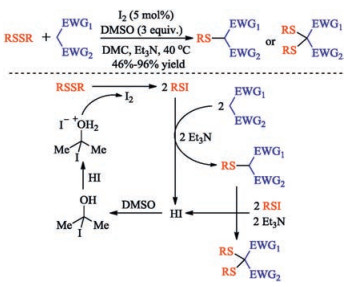
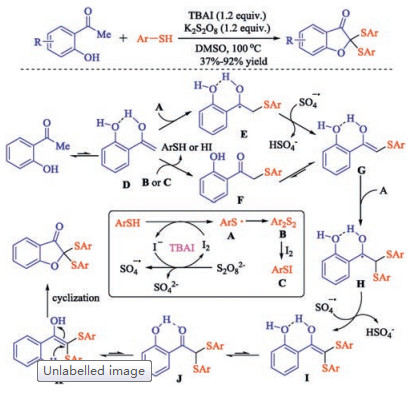
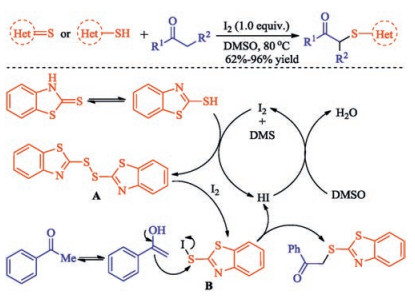
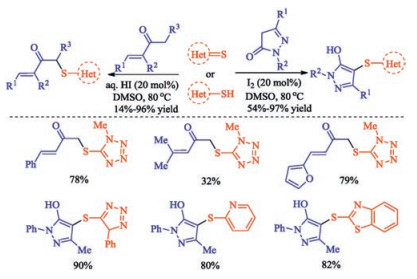
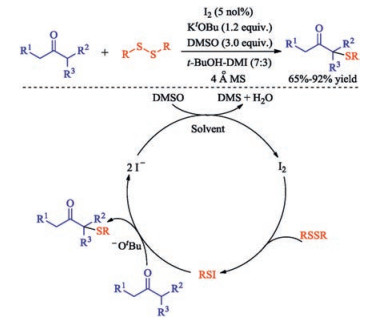
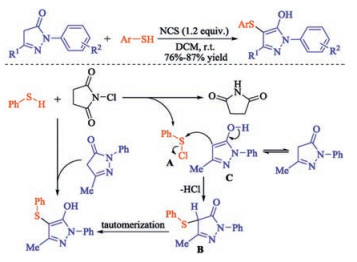
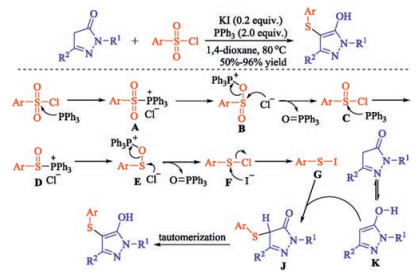
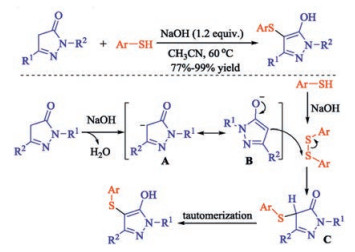
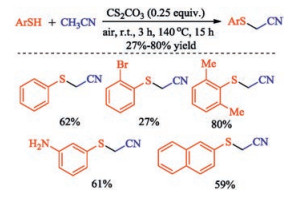
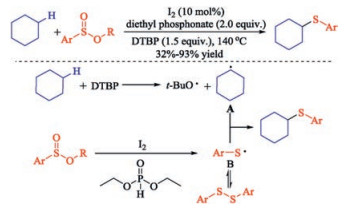
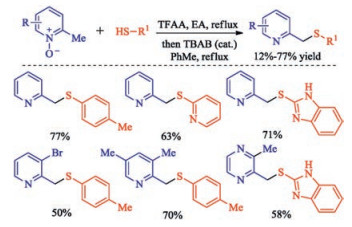
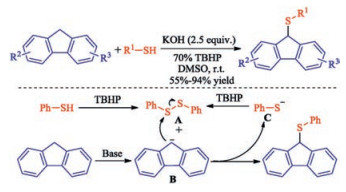
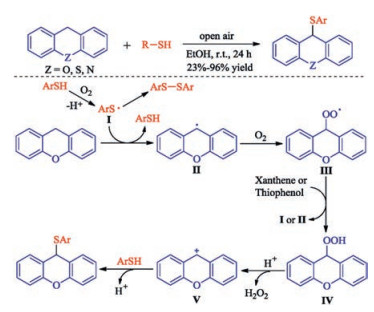
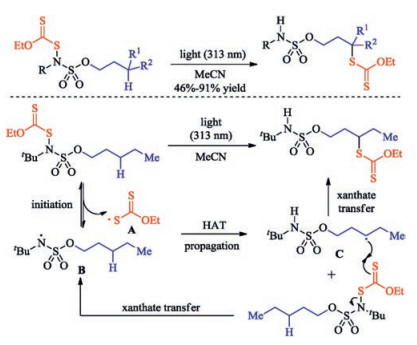
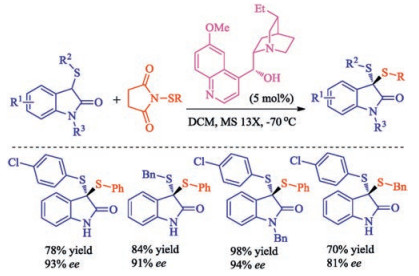
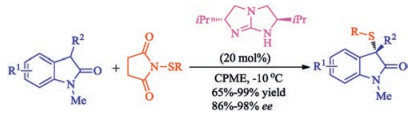
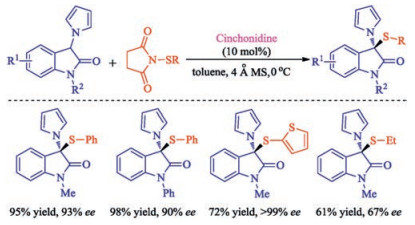
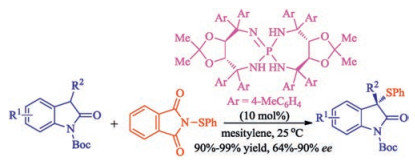
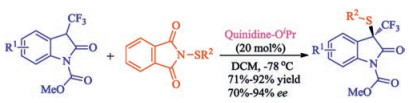
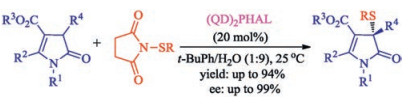
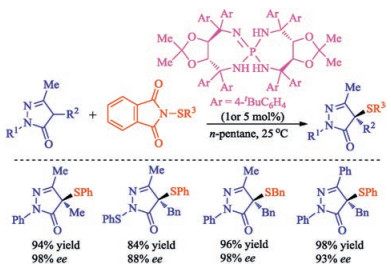
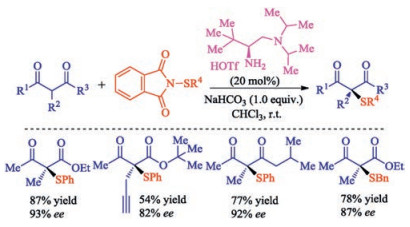
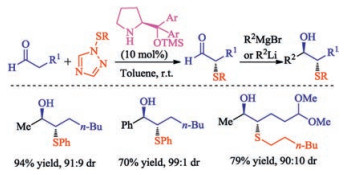
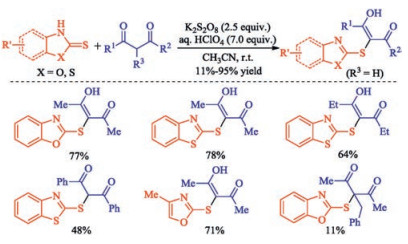
In recent years, the transition metal-free sulfenylation of C-H bond for C-S formation has been rapidly advanced and has become an eco-friendly synthetic tool for pharmacists and organic chemists. Various natural or bioactive molecules such as (hetero)arenes, olefins, carbonyl compounds, alkanes, have been employed for sulfenylating reactions. This review will focus on the recent five-year advances in C-S bond formation via direct sulfenylation of C(sp3)-H bonds under metal-free conditions and elaborate their mechanisms from a new perspective.
-
Keywords:
- Sulfenylation,
- C(sp3)-H bond,
- Metal-free,
- Reaction mechanism,
- Synthetic method
-

-
-
[1]
(a) R.J. Cremlyn, An Introduction to Organosulfur Chemistry, Wiley, New York, 1996;
(b) D. Meng, W. Chen, W. Zhao, J. Nat. Prod. 70 (2007) 824-829;
(c) M. Kvasnika, M. Urban, N.J. Dickinson, J. Sarek, Nat. Prod. Rep. 32 (2015) 1303-1330. -
[2]
M.H. Feng, B.Q. Tang, H.L. Steven, X.F. Jiang, Curr. Top. Med. Chem. 16(2016) 1200-1216. doi: 10.2174/1568026615666150915111741
-
[3]
(a) D.A. Boyd, Angew. Chem. Int. Ed. 55 (2016) 15486-15502;
(b) D. Wu, W. Pisula, M.C. Haberecht, X. Feng, K. Müllen, Org. Lett. 11 (2009) 5686-5689;
(c) S.M. Yang, J.J. Shie, J.M. Fang, S.K. Nandy, Y.Y. Chang, J. Org. Chem. 67 (2002) 52085215. -
[4]
(a) J.C. Carretero, Chem. Commun. 47(2011) 2207-2211;
(b) H. Pellisier, Chiral Sulfur Ligands in Asymmetric Catalysis, RSC Catalysis Series 2, Cambridge, 2009. -
[5]
(a) A. Kausar, S. Zulfiqar, M.I. Sarwar, Pol. Rev. 54 (2014) 185-267;
(b) A.S. Rahate, K.R. Nemade, S.A. Waghuley, Rev. Chem. Eng. 29 (2013) 471-489;
(c) N. Spassky, Phosphorus Sulfur Silicon Relat. Elem. 74 (1993) 71-92. -
[6]
(a) J.F. Hartwig, Nature 455 (2008) 314-322;
(b) Q. Lu, J. Zhang, F.L. Wei, et al., Angew. Chem. Int. Ed. 52 (2013) 7156-7159;
(c) Q.Q. Lu, J. Zhang, G.L. Zhao, et al., J. Am. Chem. Soc.135 (2013) 11481-11484;
(d) S.H. Hao, L.X. Li, D.Q. Dong, Z.L. Wang, Chin. J. Catal. 38 (2017) 1664-1667;
(e) L.H. Lu, S.J. Zhou, W.B. He, et al., Org. Biomol. Chem. 16 (2018) 9064-9068;
(f) L.Y. Xie, Y.J. Li, J. Qu, et al., Green Chem. 19 (2017) 5642-5646;
(g) F.L. Zeng, X.L. Chen, S.Q. He, et al., Org. Chem. Front. 6 (2019) 1476-1480;
(h) D. Yang, P. Sun, W. Wei, et al., Chem. -Eur. J. 24 (2018) 4423-4427;
(i) L. Penga, Z. Hua, Z. Tang, Y. Jiao, X. Xu, Chin. Chem. Lett. 30 (2019) 1481-1487. -
[7]
(a) M. Martinek, M. Korf, J. Srogl, Chem. Commun. 46 (2010) 4387-4389;
(b) S.K. Sahoo, A. Banerjee, S. Chakraborty, B.K. Patel, ACS Catal. 2 (2012) 544-551;
(c) O. Saidi, J. Marafie, A.E. Ledger, et al., J. Am. Chem. Soc. 133 (2011) 19298-19301;
(d) N. Umierski, G. Manolikakes, Org. Lett. 15 (2013) 4972-4975;
(e) Z. Wu, H. Song, X. Cui, et al., Org. Lett. 15 (2013) 1270-1273;
(f) B. Niu, L. Xu, P. Xie, et al., ACS Comb. Sci. 16 (2014) 454-458. -
[8]
(a) S.N. Zhang, S.H. Yang, L.H. Huang, et al., Chin. J. Org. Chem. 35 (2015) 2259-2274;
(b) R. Chitrakar, A. Subbarayappa, Chem. Rec. 17 (2017) 1;
(c) Y.Y. Liu, J. Xiong, L. Wei, Chin. J. Org. Chem. 37 (2017) 1667-1680;
(d) D.Q. Dong, S.H. Hao, D.S. Yang, L.X. Li, Z.L. Wang, Eur. J. Org. Chem. 2017 (2017) 6576-6592;
(e) L. Li, Y.Q. Ding, Mini-Rev. Org. Chem. 14 (2017) 407-418;
(f) R. Dalpozzo, Org. Chem. Front. 4 (2017) 2063-2078;
(g) M. Freckleton, A. Baeza, L. Benavent, R. Chinchilla, Asian. J. Org. Chem. 7 (2018) 1006-1014;
(h) C.A. Jin, Q. Xu, G.F. Feng, Y. Jin, L.Y. Zahng, Chin. J. Org. Chem. 38 (2018) 775-790. -
[9]
(a) A. Ghaderi, Tetrahedron 72 (2016) 4758-4782;
(b) K.L. Dunbar, D.H. Scharf, A. Litomska, C. Hertweck, Chem. Rev. 117 (2017) 5521-5577;
(c) J. Zhu, W.C. Yang, X.D. Wang, L. Wu, Adv. Synth. Catal. 360 (2018) 386-400;
(d) Y. Luo, Y. Ma, Z. Hou, J. Am. Chem. Soc. 140 (2018) 114-117. -
[10]
B.V. Varun, K. Gadde, K.R. Prabhu, Org. Lett. 17(2015) 2944-2947. doi: 10.1021/acs.orglett.5b01221
-
[11]
H. Cao, J. Yuan, C. Liu, X.Q. Hu, A.W. Lei, RSC Adv. 5(2015) 41493-41496. doi: 10.1039/C5RA04906G
-
[12]
Y. Jiang, J.X. Zou, L.T. Huang, et al., Org. Biomol. Chem. 16(2018) 1641-1645. doi: 10.1039/C8OB00080H
-
[13]
Q. Chen, X. Wang, C. Wen, et al., RSC Adv. 7(2017) 39758-39761. doi: 10.1039/C7RA06904A
-
[14]
Y. Liu, S.S. Badsara, Y. Liu, C. Lee, RSC Adv. 5(2015) 44299-44305. doi: 10.1039/C5RA07204B
-
[15]
R. Rahaman, N. Devi, P. Barman, Tetrahedron Lett. 56(2015) 4224-4227. doi: 10.1016/j.tetlet.2015.05.062
-
[16]
N. Devi, R. Rahaman, K. Sarma, P. Barman, Eur. J. Org. Chem. 2016(2016) 384-388. doi: 10.1002/ejoc.201501148
-
[17]
B.M. Trost, Chem. Rev. 78(1978) 363-382. doi: 10.1021/cr60314a002
-
[18]
B. Hu, Q. Zhang, S. Zhao, et al., Adv. Synth. Catal. 361(2019) 49-54. doi: 10.1002/adsc.201801138
-
[19]
Y. Siddaraju, K.R. Prabhu, Org. Lett. 18(2016) 6090-6093. doi: 10.1021/acs.orglett.6b03084
-
[20]
Y. Siddaraju, K.R. Prabhu, J. Org. Chem. 83(2018) 2986-2992. doi: 10.1021/acs.joc.7b03290
-
[21]
Y. Siddaraju, K.R. Prabhu, Org. Biomol. Chem. 15(2017) 5191-5196. doi: 10.1039/C7OB00561J
-
[22]
N. Devi, R. Rahaman, K. Sarma, T. Khan, P. Barman, Eur. J. Org. Chem. 2017(2017) 1520-1525.
-
[23]
(a) P.N. Kalaria, S.P. Satasia, J.R. Avalani, D.K. Raval, Eur. J. Med. Chem. 83 (2014) 655-664;
(b) S.C. Karad, V.B. Purohit, D.K. Raval, Eur. J. Med. Chem. 84 (2014) 51-58. -
[24]
R.D. Kamani, V.B. Purohit, R.P. Thummar, et al., ChemistrySelect 2(2017) 9670-9673. doi: 10.1002/slct.201701924
-
[25]
(a) H. Jin, W. Wang, Z. Yang, et al., Heterocycles 96 (2018) 1786-1794;
(b) X. Zhao, X. Lu, A. Wei, et al., Tetrahedron Lett. 57 (2016) 5330-5333;
(c) X. Zhao, A. Wei, X. Lu, K. Lu, Molecules 22 (2017) 1208-1219. -
[26]
X. Liu, H. Cui, D. Yang, et al., RSC Adv. 6(2016) 51830-51833. doi: 10.1039/C6RA09739A
-
[27]
Q. Chen, Y. Huang, X. Wang, et al., Tetrahedron Lett. 58(2017) 3928-3931. doi: 10.1016/j.tetlet.2017.08.067
-
[28]
(a) A.F. Vaquer, A. Frongia, F. Secci, E. Tuveri, RSC Adv. 5 (2015) 96695-96704;
(b) H.W. Noh, C. Lee, H.Y. Jang, Bull. Korean Chem. Soc. 38 (2017) 389-391;
(c) J.Q. Zhao, S.W. Luo, X.M. Zhang, et al., Tetrahedron 73 (2017) 5444-5450. -
[29]
Y. Li, F. Zhu, Z. Wang, X.F. Wu, Chem. -Asian J. 11(2016) 3503-3507. doi: 10.1002/asia.201601376
-
[30]
D. Wang, Z. Liu, Z. Wang, X. Ma, P. Yu, Green Chem. 21(2019) 157-163. doi: 10.1039/C8GC03072C
-
[31]
Y. Liu, X. Yuan, K. Su, Y. Tian, B. Chen, Eur. J. Org. Chem. 2019(2019) 1649-1652. doi: 10.1002/ejoc.201801806
-
[32]
Q. Chen, G. Yu, X. Wang, Y. Ou, Y. Huo, Green Chem. 21(2019) 798-802. doi: 10.1039/C8GC03898H
-
[33]
S.K. Ayer, J.L. Roizen, J. Org. Chem. 84(2019) 3508-3523. doi: 10.1021/acs.joc.9b00105
-
[34]
K. Liao, F. Zhou, J. Yu, W. Gao, J. Zhou, Chem. Commun. 51(2015) 16255-16258. doi: 10.1039/C5CC07010D
-
[35]
L. Huang, J. Li, Y. Zhao, et al., J. Org. Chem. 80(2015) 8933-8941. doi: 10.1021/acs.joc.5b01606
-
[36]
Y. You, Z. Wu, Z. Wang, et al., J. Org. Chem. 80(2015) 8470-8477. doi: 10.1021/acs.joc.5b01491
-
[37]
X. Gao, J. Han, L. Wang, Synthesis 48(2016) 2603-2611. doi: 10.1055/s-0035-1560435
-
[38]
Y. E, T. Yuan, L. Yin, Y. Xu, Tetrahedron Lett. 58(2017) 2521-2524. doi: 10.1016/j.tetlet.2017.05.015
-
[39]
S.J. Singha Roy, S. Mukherjee, Org. Biomol. Chem. 15(2017) 6921-6925. doi: 10.1039/C7OB01714F
-
[40]
J. Han, Y. Zhang, X.Y. Wu, H.N.C. Wong, Chem. Commun. 55(2019) 397-400. doi: 10.1039/C8CC09049A
-
[41]
L. Cui, Y. You, X. Mi, S. Luo, Org. Chem. Front. 5(2018) 2313-2316. doi: 10.1039/C8QO00496J
-
[42]
K. Nagata, D. Sano, O. Aoyama, et al., Heterocycles 92(2016) 631-635. doi: 10.3987/COM-16-13414
-
[43]
F. Rota, L. Benhamou, T.D. Sheppard, Synlett 27(2016) 33-36. doi: 10.1055/s-0035-1560769
-
[1]
-

-
-
[1]
Haoyu Tian , Xiaolin Cui , Guiwei Yao , Wenyan Wei , Junchao Lu , Senyao Zheng , Xingjian Wang , Xun Chen , Guangkuan Zhao , Dulin Kong . The sulfenylation of enamine esters with heterocyclic thiols or disulfides in water and application to DNA-compatible chemistry. Chinese Chemical Letters, 2025, 36(12): 111006-. doi: 10.1016/j.cclet.2025.111006
-
[2]
Huixin Chen , Chen Zhao , Hongjun Yue , Guiming Zhong , Xiang Han , Liang Yin , Ding Chen . Unraveling the reaction mechanism of high reversible capacity CuP2/C anode with native oxidation POx component for sodium-ion batteries. Chinese Chemical Letters, 2025, 36(1): 109650-. doi: 10.1016/j.cclet.2024.109650
-
[3]
Tong Li , Leping Pan , Yan Zhang , Jihu Su , Kai Li , Kuiliang Li , Hu Chen , Qi Sun , Zhiyong Wang . Electrochemical construction of 2,5-diaryloxazoles via N–H and C(sp3)-H functionalization. Chinese Chemical Letters, 2024, 35(4): 108897-. doi: 10.1016/j.cclet.2023.108897
-
[4]
Ao Sun , Zipeng Li , Shuchun Li , Xiangbao Meng , Zhongtang Li , Zhongjun Li . Stereoselective synthesis of α-3-deoxy-D-manno-oct-2-ulosonic acid (α-Kdo) derivatives using a C3-p-tolylthio-substituted Kdo fluoride donor. Chinese Chemical Letters, 2025, 36(3): 109972-. doi: 10.1016/j.cclet.2024.109972
-
[5]
Chunhua Ma , Mengjiao Liu , Siyu Ouyang , Zhenwei Cui , Jingjing Bi , Yuqin Jiang , Zhiguo Zhang . Metal-free construction of diverse 1,2,4-triazolo[1,5-a]pyridines on water. Chinese Chemical Letters, 2025, 36(1): 109755-. doi: 10.1016/j.cclet.2024.109755
-
[6]
Lei Li , Guang Yang , Tianbai Xiong , Tingzhu Duan , Jia Wang , Xin Wang . Metal-free click polymerization of thiols and chalcone-derived internal olefins in air to prepare functional clusteroluminescent polythioethers for dual-response fluorescent probe. Chinese Chemical Letters, 2025, 36(11): 111374-. doi: 10.1016/j.cclet.2025.111374
-
[7]
Linyu Zhu , Xu Tian , Guang Shi , Wenchi Zhang , Peisong Tang , Mohamed Bououdina , Sajjad Ali , Pengfei Xia . Assembling 3D cross-linked network by carbon nitride nanowires for visible-light photocatalytic H2 evolution from dyestuffs wastewater. Chinese Chemical Letters, 2025, 36(12): 111088-. doi: 10.1016/j.cclet.2025.111088
-
[8]
Jiaming Li , Na Xu , Yafei Zhang , Hongjun Dong , Chunmei Li . Research progress of heterogeneous photocatalyst for H2O2 production: A mini review. Chinese Chemical Letters, 2025, 36(11): 110470-. doi: 10.1016/j.cclet.2024.110470
-
[9]
Qin Cheng , Ming Huang , Qingqing Ye , Bangwei Deng , Fan Dong . Indium-based electrocatalysts for CO2 reduction to C1 products. Chinese Chemical Letters, 2024, 35(6): 109112-. doi: 10.1016/j.cclet.2023.109112
-
[10]
Pin Cui , Ying Tang , Jie Yu , Zhen Yang , Shouhua Yang , Boqin Li , Gang Wang , Huan Pang , Feng Yu . Bimetallic ZnFe–NC prepared using microchannel reactor for oxygen reduction reaction and mechanism research. Chinese Chemical Letters, 2025, 36(9): 110303-. doi: 10.1016/j.cclet.2024.110303
-
[11]
Zhigang Zeng , Changzhou Liao , Lei Yu . Molecules for COVID-19 treatment. Chinese Chemical Letters, 2024, 35(7): 109349-. doi: 10.1016/j.cclet.2023.109349
-
[12]
Kai Zhu , Lei Yang , Yang Yang , Yanqi Wu , Fengzhi Zhang . Recent advances toward the catalytic enantioselective synthesis of planar chiral cyclophanes. Chinese Chemical Letters, 2025, 36(7): 110678-. doi: 10.1016/j.cclet.2024.110678
-
[13]
Hefei Yang , Le-Cheng Wang , Xiao-Feng Wu . Sustainable carbonylative transformation of alkyl iodides to amides via crosslinking of EDA and XAT. Chinese Chemical Letters, 2025, 36(9): 110843-. doi: 10.1016/j.cclet.2025.110843
-
[14]
Tingting Liu , Pengfei Sun , Wei Zhao , Yingshuang Li , Lujun Cheng , Jiahai Fan , Xiaohui Bi , Xiaoping Dong . Magnesium doping to improve the light to heat conversion of OMS-2 for formaldehyde oxidation under visible light irradiation. Chinese Chemical Letters, 2024, 35(4): 108813-. doi: 10.1016/j.cclet.2023.108813
-
[15]
Chunrui Zhao , Tianren Li , Jiage Li , Yansong Liu , Zian Fang , Xinyu Wang , Mingxin Huo , Shuangshi Dong , Mingyu Li . Doped cobalt for simultaneously promoting active (001) facet exposure of MIL-68(In) and acting as reactive sites in peroxymonosulfate-mediated photocatalytic decontamination. Chinese Chemical Letters, 2025, 36(5): 110201-. doi: 10.1016/j.cclet.2024.110201
-
[16]
Xin Zhou , Xuejia Li , Yujia Xiang , Heng Zhang , Chuanshu He , Zhaokun Xiong , Wei Li , Peng Zhou , Hongyu Zhou , Yang Liu , Bo Lai . The application of low-valent sulfur oxy-acid salts in advanced oxidation and reduction processes: A review. Chinese Chemical Letters, 2025, 36(9): 110664-. doi: 10.1016/j.cclet.2024.110664
-
[17]
Jian Han , Li-Li Zeng , Qin-Yu Fei , Yan-Xiang Ge , Rong-Hui Huang , Fen-Er Chen . Recent advances in remote C(sp3)–H functionalization via chelating group-assisted metal-catalyzed chain-walking reaction. Chinese Chemical Letters, 2024, 35(11): 109647-. doi: 10.1016/j.cclet.2024.109647
-
[18]
Guoju Guo , Xufeng Li , Jie Ma , Yongjia Shi , Jian Lv , Daoshan Yang . Photocatalyst/metal-free sequential C–N/C–S bond formation: Synthesis of S-arylisothioureas via photoinduced EDA complex activation. Chinese Chemical Letters, 2024, 35(11): 110024-. doi: 10.1016/j.cclet.2024.110024
-
[19]
Tao Zhou , Jing Zhou , Yunyun Liu , Jie-Ping Wan , Fen-Er Chen . Transition metal-free tunable synthesis of 3-(trifluoromethylthio) and 3-trifluoromethylsulfinyl chromones via domino C–H functionalization and chromone annulation of enaminones. Chinese Chemical Letters, 2024, 35(11): 109683-. doi: 10.1016/j.cclet.2024.109683
-
[20]
Jianhui Yin , Wenjing Huang , Changyong Guo , Chao Liu , Fei Gao , Honggang Hu . Tryptophan-specific peptide modification through metal-free photoinduced N-H alkylation employing N-aryl glycines. Chinese Chemical Letters, 2024, 35(6): 109244-. doi: 10.1016/j.cclet.2023.109244
-
[1]
Metrics
- PDF Downloads(27)
- Abstract views(2042)
- HTML views(62)

 Login In
Login In




 DownLoad:
DownLoad: