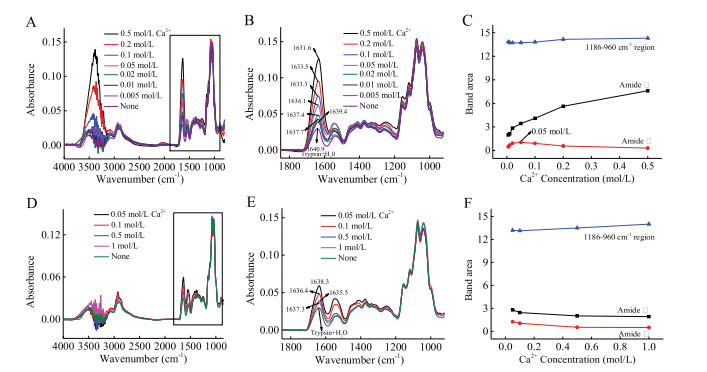
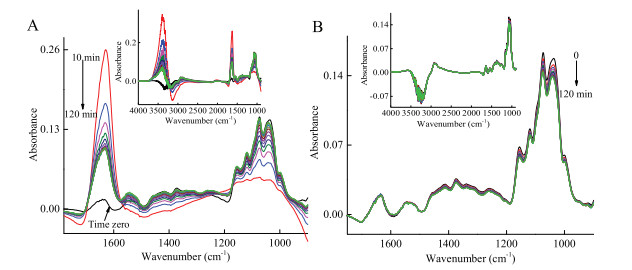
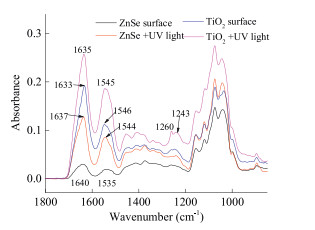
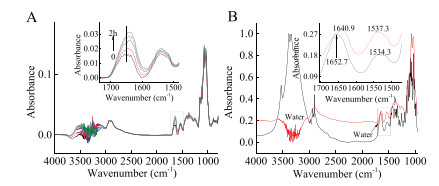
-
[1]
Jun Zhang
, Zhiyao Zheng
, Can Zhu
. Stereochemical editing: Catalytic racemization of secondary alcohols and amines. Chinese Chemical Letters,
2024, 35(5): 109160-.
doi: 10.1016/j.cclet.2023.109160
-
[2]
Jiakun Bai
, Junhui Jia
, Aisen Li
. An elastic organic crystal with piezochromic luminescent behavior. Chinese Journal of Structural Chemistry,
2024, 43(6): 100323-100323.
doi: 10.1016/j.cjsc.2024.100323
-
[3]
Bingyang Lu
, Dehui Wang
, Junchang Guo
, Yang Shen
, Qian Feng
, Jinlong Yang
, Xiao Han
, Huali Yu
, Luohuizi Li
, Jiaxin Liu
, Jing Luo
, Huan Liu
, Zhongwei Zhang
, Xu Deng
. High-efficiency exudates drainage of anti-adhesion dressings for chronic wound. Chinese Chemical Letters,
2025, 36(4): 110601-.
doi: 10.1016/j.cclet.2024.110601
-
[4]
Mengyu Chen
, Qinglin Zhou
, Tianyun Qin
, Ningyao Sun
, Yuxi Chen
, Yuwei Gong
, Xingyi Li
, Jinsong Liu
. An ionic liquid-reinforced gelatin hydrogel with strong adhesion, antibacterial and anti-inflammatory properties for treating oral ulcers. Chinese Chemical Letters,
2025, 36(7): 110441-.
doi: 10.1016/j.cclet.2024.110441
-
[5]
Yuhan Wu
, Qing Zhao
, Zhijie Wang
. Layered vanadium oxides: Promising cathode materials for calcium-ion batteries. Chinese Journal of Structural Chemistry,
2024, 43(5): 100271-100271.
doi: 10.1016/j.cjsc.2024.100271
-
[6]
Yunzhe Du
, Siliu Cheng
, Shuyi Li
, Junping Niu
, Yuan Gou
, Ligang Yan
, Tian-Xing Zhang
, Ruijun Xie
, Limin Han
, Tiezheng Jia
, Ning Zhu
. C2-Transformation of calcium carbide trigered by hydrogen sulfide. Chinese Chemical Letters,
2025, 36(12): 111233-.
doi: 10.1016/j.cclet.2025.111233
-
[7]
Yuan Liu
, Boyang Wang
, Yaxin Li
, Weidong Li
, Siyu Lu
. Understanding excitonic behavior and electroluminescence light emitting diode application of carbon dots. Chinese Chemical Letters,
2025, 36(2): 110426-.
doi: 10.1016/j.cclet.2024.110426
-
[8]
Yuan Dong
, Mutian Ma
, Zhenyang Jiao
, Sheng Han
, Likun Xiong
, Zhao Deng
, Yang Peng
. Effect of electrolyte cation-mediated mechanism on electrocatalytic carbon dioxide reduction. Chinese Chemical Letters,
2024, 35(7): 109049-.
doi: 10.1016/j.cclet.2023.109049
-
[9]
Yinghui Xia
, Yixi Lin
, Zhenming Xu
. Cation potential guiding structural regulation of lithium halide superionic conductors. Chinese Journal of Structural Chemistry,
2025, 44(3): 100448-100448.
doi: 10.1016/j.cjsc.2024.100448
-
[10]
Wenjie Ma
, Yakun Tang
, Yue Zhang
, Lang Liu
, Bin Tang
, Dianzeng Jia
, Yuliang Cao
. Cation-disordered Li2FeTiO4 nanoparticles with multiple cation and anion redox for symmetric lithium-ion batteries. Chinese Chemical Letters,
2025, 36(9): 110346-.
doi: 10.1016/j.cclet.2024.110346
-
[11]
Xinyu You
, Xin Zhang
, Shican Jiang
, Yiru Ye
, Lin Gu
, Hexun Zhou
, Pandong Ma
, Jamal Ftouni
, Abhishek Dutta Chowdhury
. Efficacy of Ca/ZSM-5 zeolites derived from precipitated calcium carbonate in the methanol-to-olefin process. Chinese Journal of Structural Chemistry,
2024, 43(4): 100265-100265.
doi: 10.1016/j.cjsc.2024.100265
-
[12]
Shiyu Pan
, Bo Cao
, Deling Yuan
, Tifeng Jiao
, Qingrui Zhang
, Shoufeng Tang
. Complexes of cupric ion and tartaric acid enhanced calcium peroxide Fenton-like reaction for metronidazole degradation. Chinese Chemical Letters,
2024, 35(7): 109185-.
doi: 10.1016/j.cclet.2023.109185
-
[13]
Haoyu Luo
, Jinsong Chen
, Mengfei Luo
, Hui Ma
, Shengyan Pu
. Heterogeneous Fenton catalytic degradation of nitrobenzene by controlled-release nano calcium peroxide. Chinese Chemical Letters,
2025, 36(6): 110367-.
doi: 10.1016/j.cclet.2024.110367
-
[14]
Yiyang Zhang
, Guangshu Yuan
, Xiangkun Meng
, Xu Zhang
, Lei Yu
. Promoting the catalytic activities of polyanilines for L-lactic acid condensation by calcium-doping: A biocompatible strategy. Chinese Chemical Letters,
2025, 36(12): 111069-.
doi: 10.1016/j.cclet.2025.111069
-
[15]
Hongdao LI
, Shengjian ZHANG
, Hongmei DONG
. Magnetic relaxation and luminescent behavior in nitronyl nitroxide-based annuluses of rare-earth ions. Chinese Journal of Inorganic Chemistry,
2024, 40(5): 972-978.
doi: 10.11862/CJIC.20230411
-
[16]
Yan Zou
, Yin-Shuang Hu
, Deng-Hui Tian
, Hong Wu
, Xiaoshu Lv
, Guangming Jiang
, Yu-Xi Huang
. Tuning the membrane rejection behavior by surface wettability engineering for an effective water-in-oil emulsion separation. Chinese Chemical Letters,
2024, 35(6): 109090-.
doi: 10.1016/j.cclet.2023.109090
-
[17]
Siwei Wang
, Wei-Lei Zhou
, Yong Chen
. Cucurbituril and cyclodextrin co-confinement-based multilevel assembly for single-molecule phosphorescence resonance energy transfer behavior. Chinese Chemical Letters,
2024, 35(12): 110261-.
doi: 10.1016/j.cclet.2024.110261
-
[18]
Yinling HOU
, Jia JI
, Hong YU
, Xiaoyun BIAN
, Xiaofen GUAN
, Jing QIU
, Shuyi REN
, Ming FANG
. A rhombic Dy4-based complex showing remarkable single-molecule magnet behavior. Chinese Journal of Inorganic Chemistry,
2025, 41(3): 605-612.
doi: 10.11862/CJIC.20240251
-
[19]
Qihou Li
, Jiamin Liu
, Fulu Chu
, Jinwei Zhou
, Jieshuangyang Chen
, Zengqiang Guan
, Xiyun Yang
, Jie Lei
, Feixiang Wu
. Coordinating lithium polysulfides to inhibit intrinsic clustering behavior and facilitate sulfur redox conversion in lithium-sulfur batteries. Chinese Chemical Letters,
2025, 36(5): 110306-.
doi: 10.1016/j.cclet.2024.110306
-
[20]
Zhaoyu Jin
, Renjun Guan
, Xin Li
, Dunyi Yuan
, Panpan Li
. Advanced characterization techniques for understanding electrocatalytic behavior of oxidized nitrogen waste upcycling processes. Chinese Chemical Letters,
2025, 36(7): 110506-.
doi: 10.1016/j.cclet.2024.110506

 Login In
Login In






 DownLoad:
DownLoad: