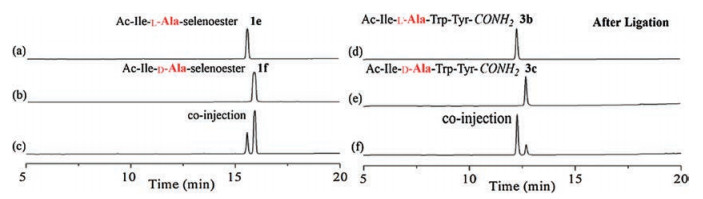
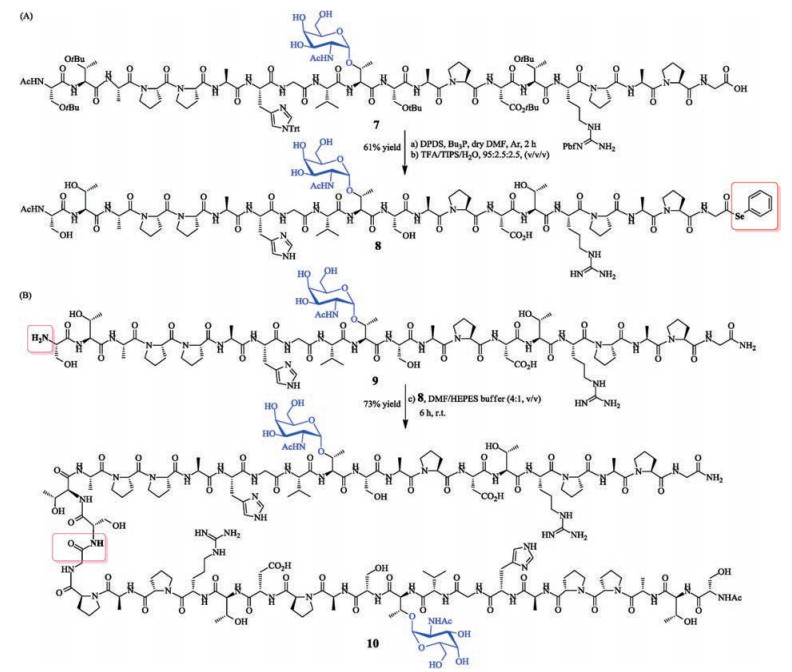
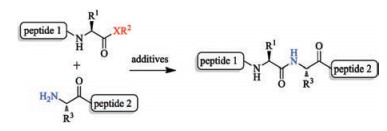
-
[1]
P.E. Dawson, T.W. Muir, I. Clark-Lewis, S.B. Kent, Science 266(1994) 776-779.
doi: 10.1126/science.7973629
-
[2]
(a) C. P. Hackenberger, D. Schwarzer, Angew. Chem. Int. Ed. 47 (2008) 10030-10074;
(b) C. Unverzagt, Y. Kajihara, Chem. Soc. Rev. 42 (2013) 4408-4420;
(c) C. T. Wong, C. L. Tung, X. Li, Mol. Biosyst. 9 (2013) 826-833;
(d) H. Hojo, Curr. Opin. Struct. Boil. 26 (2014) 16-23;
(e) L. R. Malins, R. J. Payne, Aust. J. Chem. 68 (2015) 521-537;
(f) Y. C. Huang, G. M. Fang, L. Liu, Natl. Sci. Rev. 3 (2016) 107-116;
(g) S. Bondalapati, M. Jbara, A. Brik, Nat. Chem. 8 (2016) 407;
(h) H. Li, S. Dong, Sci. China Chem. 60 (2017) 201-213;
(i) V. Agouridas, O. El Mahdi, M. Cargoët, O. Melnyk, Bioorg. Med. Chem. 25 (2017) 4938-4945;
(j) J. Yang, J. Zhao, Sci. China Chem. 61 (2018) 97-112;
(k) S. Kent, Bioorg. Med. Chem. 25 (2017) 4926-4937.
-
[3]
(a) J. B. Blanco-Canosa, B. Nardone, F. Albericio, P. E. Dawson, J. Am. Chem. Soc. 137 (2015) 7197-7209;
(b) Z. Wang, W. Xu, L. Liu, T. F. Zhu, Nat. Chem. 8 (2016) 698-704;
(c) Y. Gui, L. Qiu, Y. Li, H. Li, S. Dong, J. Am. Chem. Soc. 138 (2016) 4890-4899;
(d) A. M. Levinson, J. H. McGee, A. G. Roberts, et al., J. Am. Chem. Soc. 139 (2017) 7632-7639;
(e) S. Tang, C. Zuo, D. L. Huang, et al., Nat. Protoc. 12 (2017) 2554-2569;
(f) H. Liu, Y. Zhang, R. Wei, G. Andolina, X. Li, J. Am. Chem. Soc. 139 (2017) 13420-13428;
(g) K. Jin, T. Li, H. Y. Chow, H. Liu, X. Li, Angew. Chem. 129 (2017) 14799-14803;
(h) L. J. Liang, Y. Si, S. Tang, et al., Chin. Chem. Lett. 29 (2018) 1155-1159.
-
[4]
(a) D. Kemp, Z. W. Bernstein, G. N. McNeil, J. Org. Chem. 39 (1974) 2831-2835;
(b) D. Kemp, S. L. H. Choong, J. Pekaar, J. Org. Chem. 39 (1974) 3841-3847;
(c) G. Chen, Q. Wan, Z. Tan, et al., Angew. Chem Int. Ed. 46 (2007) 7383-7387;
(d) C. Xu, J. Xu, H. Liu, X. Li, Chin. Chem. Lett. 29 (2018) 1119-1122.
-
[5]
(a) J. Blake, Int. J. Pept. Protein Res. 17 (1981) 273-274;
(b) S. Aimoto, Biopolymers 51 (1999) 247-265;
(c) R. J. Payne, S. Ficht, W. A. Greenberg, C. H. Wong, Angew. Chem. Int. Ed. 47 (2008) 4411-4415;
(d) C. L. Tung, C. T. T. Wong, X. Li, Org. Biomol. Chem. 13 (2015) 6922-6926.
-
[6]
(a) D. Crich, I. Sharma, Angew. Chem. Int. Ed. 48 (2009) 2355-2358;
(b) P. Wang, S. J. Danishefsky, J. Am. Chem. Soc. 132 (2010) 17045-17051;
(c) S. M. Mali, S. V. Jadhav, H. N. Gopi, Chem. Commun. 48 (2012) 7085-7087.
-
[7]
(a) M. D. Gieselman, L. Xie, W. A. Van Der Donk, Org. Lett. 3 (2001) 1331-1334;
(b) R. Quaderer, A. Sewing, D. Hilvert, Helv. Chim. Acta 84 (2001) 1197-1206;
(c) R. J. Hondal, B. L. Nilsson, R. T. Raines, J. Am. Chem. Soc. 123 (2001) 5140-5141;
(d) N. Metanis, E. Keinan, P. E. Dawson, Angew. Chem. Int. Ed. 49 (2010) 7049-7053;
(e) S. Dery, P. S. Reddy, L. Dery, R. Mousa, R. N. Dardashti, N. Metanis, Chem. Sci. 6 (2015) 6207-6212;
(f) L. R. Malins, N. J. Mitchell, S. McGowan, R. J. Payne, Angew. Chem. Int. Ed. 54 (2015) 12716-12721.
-
[8]
(a) T. Durek, P. F. Alewood, Angew. Chem. Int. Ed. 50 (2011) 12042-12045;
(b) A. Ghassemian, X. Vila-Farrés, P. F. Alewood, T. Durek, Bioorg. Med. Chem. 21 (2013) 3473-3478;
(c) L. R. Malins, N. J. Mitchell, R. J. Payne, J. Pept. Sci. 20 (2014) 64-77;
(d) M. Raj, H. Wu, S. L. Blosser, M. A. Vittoria, P. S. Arora, J. Am. Chem. Soc. 137 (2015) 6932-6940;
(e) L. Raibaut, H. Drobecq, O. Melnyk, Org. Lett. 17 (2015) 3636-3639;
(f) N. J. Mitchell, L. R. Malins, X. Liu, etal., J. Am. Chem. Soc. 137 (2015)14011-14014;
(g)A. Temperini, F. Piazzolla, L. Minuti, M. Curini, C. Siciliano, J. Org. Chem. 82 (2017) 4588-4603;
(h) C. C. Hanna, S. S. Kulkarni, E. E. Watson, B. Premdjee, R. J. Payne, Chem. Commun. 53 (2017) 5424-5427;
(i) N. J. Mitchell, J. Sayers, S. S. Kulkarni, et al., Chemistry 2 (2017) 703-715;
(j) T. Takei, T. Andoh, T. Takao, H. Hojo, Angew. Chem. 129 (2017) 15914-15917.
-
[9]
(a) X. F. Gao, J. J. Du, Z. Liu, J. Guo, Org. Lett. 18 (2016) 1166-1169;
(b) J. J. Du, X. F. Gao, L. M. Xin, et al., Org. Lett. 18 (2016) 4828-4831;
(c) X. G. Yin, X. F. Gao, J. J. Du, et al., Org. Lett 18 (2016) 5796-5799;
(d) X. G. Yin, X. Z. Chen, W. M. Sun, et al., Org. Lett. 19 (2017) 456-459;
(e) J. Wang, R. Y. Zhang, Y. C. Wang, et al., Synlett 28 (2017) 1934-1938;
(f) Z. Liu, J. Guo, Carbohydr. Res. 452 (2017) 78-90.
-
[10]
(a) Taylor-Papadimitriou, J. Burchell, D. Miles, M. Dalziel, Biochim. Biophys. Acta 1455 (1999) 301-313;
(b) V. Apostolopoulos, L. Stojanovska, S. E. Gargosky, Cell. Mol. Life Sci. 72 (2015) 4475-4500.
-
[11]
(a) Z. H. Huang, L. Shi, J. W. Ma, et al., J. Am. Chem. Soc. 134 (2012) 8730-8733;
(b) N. Gaidzik, U. Westerlind, H. Kunz, Chem. Soc. Rev. 42 (2013) 4421-4442;
(c) H. Cai, Z. Y. Sun, M. S. Chen, et al., Angew. Chem. Int. Ed. 53 (2014) 1699-1703;
(d) M. Movahedin, T. M. Brooks, N. T. Supekar, et al., Glycobiology 27 (2017) 677-687.
-
[12]
C. Xu, H.Y. Lam, Y. Zhang, X. Li, Chem. Commun. 49(2013) 6200-6202.
doi: 10.1039/c3cc42573h

 Login In
Login In






 DownLoad:
DownLoad: