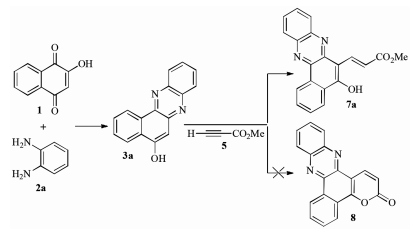
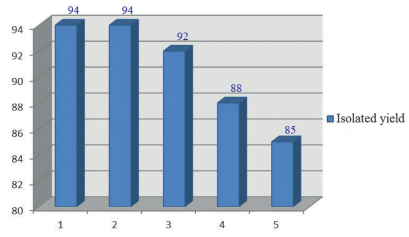
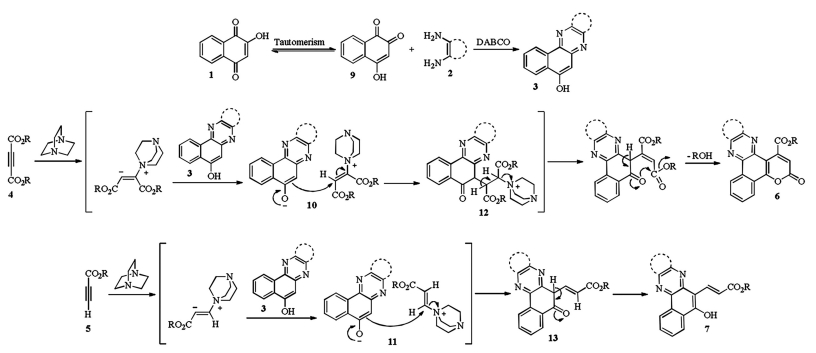
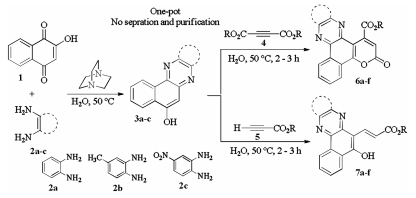
-
[1]
Yu J., Shi F., Gong L.Z.. Brønsted-acid-catalyzed asymmetric multicomponent reactions for the facile synthesis of highly enantioenriched structurally diverse nitrogenous heterocycles[J]. Acc. Chem. Res.,
2011(44):1156-1171.
-
[2]
Singh M.S., Nandi G.C., Chanda T.. β-Oxodithioesters:a new frontier for diverse heterocyclic architectures[J]. RSC Adv.,
2013(3):14183-14198.
-
[3]
Xu Z., Ayaz M., Cappelli A.A., Hulme C.. General one-pot, two-step protocol accessing a range of novel polycyclic heterocycles with high skeletal diversity[J]. ACS Comb. Sci.,
2012(14):460-464.
-
[4]
Jiang B., Tu S.J., Kaur P., Wever W., Li G.. Four-component domino reaction leading to multifunctionalized quinazolines[J]. J. Am. Chem. Soc.,
2009(131):11660-11661.
-
[5]
Knapp J.M., Zhu J.S., Tantillo D.J., Kurth M.J.. Multicomponent assembly of highly substituted indoles by dual palladium-catalyzed coupling reactions[J]. Angew. Chem. Int. Ed.,
2012(51):10588-10591.
-
[6]
Li M., Cao H., Wang Y., Lv X.L., Wen L.R.. One-pot multicomponent cascade reaction of N S-ketene acetal:solvent-free synthesis of imidazo[1, 2-a] thiochromeno[3.2-e]pyridines[J]. Org. Lett.,
2012(14):3470-3473.
-
[7]
Khurana J.M., Chaudhary A., Lumb A., Nand B.. An expedient four-component domino protocol for the synthesis of novel benzo[a] phenazine annulated heterocycles and their photophysical studies[J]. Green Chem.,
2012(14):2321-2327.
-
[8]
McNulty J., Das P.. Highly stereoselective and general synthesis of (E)-stilbenes and alkenes by means of an aqueous Wittig reaction[J]. Eur. J. Org. Chem.,
2009:4031-4035.
-
[9]
McNulty J., Das P., McLeod D.. Microwave-assisted, aqueous wittig reactions:organic-solvent-and protecting-group-free chemoselective synthesis of functionalized alkenes[J]. Chem. Eur. J.,
2010(16):6756-6760.
-
[10]
Narayan S., Muldoon J., Finn M.G.. On water:unique reactivity of organic compounds in aqueous suspension[J]. Angew. Chem. Int. Ed.,
2005(44):3275-3279.
-
[11]
Smith M.B., March J.. March's advanced organic chemistry:reactions, mechanisms, and structure[J]. John Wiley & Sons,
2007.
-
[12]
R. Breslow, in: M. Lindstrom (Ed. ), In Organic Reactions in Water, Blackwell, Oxford, 2007, pp. 1-28.
-
[13]
Rideout D.C., Breslow R.. Hydrophobic acceleration of Diels-Alder reactions[J]. J. Am. Chem. Soc.,
1980(102):7816-7817.
-
[14]
Breslow R.. Hydrophobic effects on simple organic reactions in water[J]. Acc. Chem. Res.,
1991(24):159-164.
-
[15]
Dömling A., Ugi I.. Multicomponent reactions with isocyanides[J]. Angew. Chem. Int. Ed.,
2000(39):3168-3210.
-
[16]
Dömling A.. Recent developments in isocyanide based multicomponent reactions in applied chemistry[J]. Chem. Rev.,
2006(106):17-89.
-
[17]
de Witte N.V., Stoppani A.O., Dubin M.. 2-Phenyl-β-lapachone can affect mitochondrial function by redox cycling mediated oxidation[J]. Arch. Biochem. Biophys.,
2004(432):129-135.
-
[18]
Lopez L.M., Pellegrino de Iraldi A., Carrizo P.H., Dubin M., Stoppani A.O.M.. Effect of the lipophilic o-naphthoquinone CG 10-248 on rat liver mitochondria structure and function[J]. Biocell,
2002(26):237-245.
-
[19]
Ferreira S.B., Salom K., da Silva F.C.. Synthesis and anti-trypanosoma cruzi activity of β-lapachone analogues[J]. Eur. J. Med. Chem.,
2011(46):3071-3077.
-
[20]
Bloxham J., Dell C.P., Smith C.. Preparation of some new benzylidenemalononitriles by an SNAr reaction:application to naphtho[1, 2-b] pyran synthesis[J]. Heterocycles,
1994(38):399-408.
-
[21]
Makgatho M.E., Anderson R., sullivan J.F. O'. Tetramethylpiperidinesubstituted phenazines as novel anti-plasmodial agents[J]. Drug Develop. Res.,
2000(50):195-202.
-
[22]
de Andrade-Neto V.F., Goulart M.O., da Silva Filho J.F.. β-lapachone and its derivatives against Plasmodium falciparum in vitro and Plasmodium berghei in vivo[J]. Bioorg. Med. Chem. Lett.,
2004(14):1145-1149.
-
[23]
Neves-Pinto C., Malta V., Pinto M.. A trypanocidal phenazine derived from β-lapachone[J]. J. Med. Chem.,
2002(45):2112-2115.
-
[24]
Cartwright D.K., Chilton W.S., Benson D.M.. Benson, Pyrrolnitrin and phenazine production by Pseudomonas cepacia strain 5.5 B, a biocontrol agent of Rhizoctonia solani[J]. Appl. Microbiol. Biotechnol.,
1995(43):211-216.
-
[25]
Ligon J.M., Hill D.S., Hammer P.E.. Natural products with antifungal activity from Pseudomonas biocontrol bacteria[J]. Pest. Manag. Sci.,
2000(56):688-695.
-
[26]
Muller M., Sorrell T.C.. Inhibition of the human platelet cyclooxygenase response by the naturally occurring phenazine derivative, 1-hydroxyphenazine[J]. Prostaglandins,
1995(50):301-311.
-
[27]
Rewcastle G.W., Denny W.A., Baguley B.C.. Potential antitumor agents. 51. Synthesis and antitumor activity of substituted phenazine-1-carboxamides[J]. J. Med. Chem.,
1987(30):843-851.
-
[28]
Lee H.J., Kim J.S., Park S.Y.. Synthesis and cytotoxicity evaluation of 6, 11-dihydro-pyridazo-and 6, 11-dihydro-pyrido[2, 3-b]phenazine-6, 11-diones[J]. Bioorg. Med. Chem.,
2004(12):1623-1628.
-
[29]
Kim J.S., Rhee H.K., Park H.J.. Synthesis of 6-chloroisoquinoline-58-diones and pyrido[3, 4-b]phenazine-5, 12-diones and evaluation of their cytotoxicity and DNA topoisomerase Ⅱ inhibitory activity[J]. Bioorg. Med. Chem.,
2007(15):451-457.
-
[30]
Maghsoodlou M.T., Habibi S.M., Khorassani -, Moradi A.. One-pot threecomponent synthesis of functionalized spirolactones by means of reaction between aromatic ketones, dimethyl acetylenedicarboxylate, and Nheterocycles[J]. Tetrahedron,
2011(67):8492-8495.
-
[31]
Dastoorani P., Maghsoodlou M.T., Khalilzadeh M.A., Sarina E.. Synthesis of new dibenzofuran derivatives via Diels-Alder reaction of euparin with activated acetylenic esters[J]. Tetrahedron Lett.,
2016(57):314-316.
-
[32]
Maghsoodlou M.T., Khorassani S.M.H., Heydari R.. Highly stereoselective construction of functionalized cyclopropanes from the reaction between acetylenic esters and C-H acids in the presence of triphenylarsine[J]. Tetrahedron Lett.,
2009(50):4439-4442.
-
[33]
Rostami Charati F., Maghsoodlou M.T., Habibi-Khorassani S.M., Makha M.. Green diastereoselective synthesis of highly functionalised trifluoromethylated γ-lactone phosphonate esters bearing a thioester or ketothiophene[J]. Tetrahedron Lett.,
2008(49):343-347.
-
[34]
Doostmohammadi R., Maghsoodlou M.T., Hazeri N., Habibi-Khorassani S.M.. Acetic acid as an efficient catalyst for the one-pot preparation of 3, 4, 5-substituted furan-2(5H)-ones[J]. Res. Chem. Intermed.,
2013(39):4061-4066.
-
[35]
Doostmohammadi R., Maghsoodlou M.T., Hazeri N., Habibi-Khoras S.M.. An efficient one-pot multi-component synthesis of 3, 4, 5-substituted furan-2(5H)-ones catalyzed by tetra-n-butylammonium bisulphate[J]. Chin. Chem. Lett.,
2013(24):901-903.
-
[36]
Sajadikhah S.S., Maghsoodlou M.T., Hazeri N.. A simple and efficient approach to one-pot synthesis of mono-and bis-N-aryl-3-aminodihydropyrrol-2-one-4-carboxylates catalyzed by InCl3[J]. Chin. Chem. Lett.,
2014(25):58-60.
-
[37]
Bita B.. 1, 4-Diazabicyclo[2.2.2] octane (DABCO) as a useful catalyst in organic synthesis[J]. Eur. J. Chem.,
2010(1):54-60.

 Login In
Login In






 DownLoad:
DownLoad: