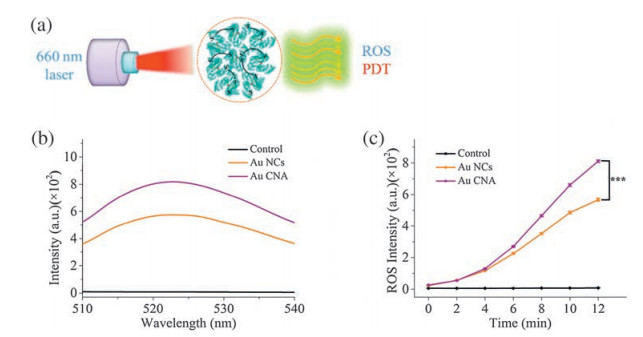
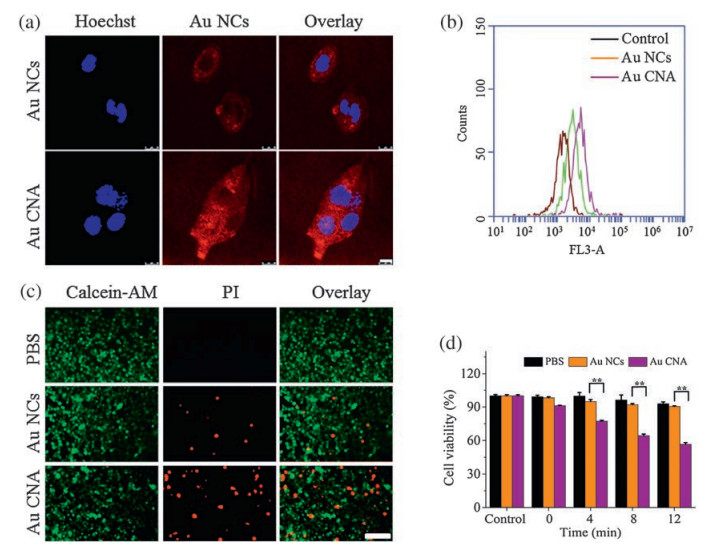
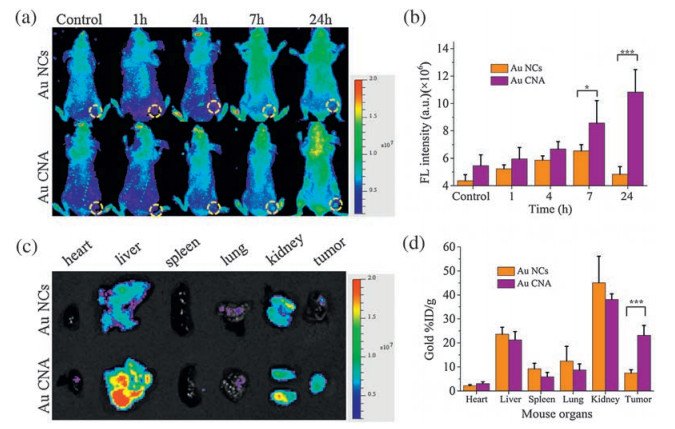
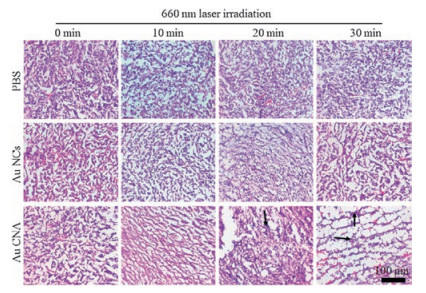
-
[1]
Mathew A., Pradeep T.. Noble metal clusters: applications in energy, environment, and biology[J]. Part. Part. Syst. Charact.,
2014,31:1017-1053.
doi: 10.1002/ppsc.v31.10
-
[2]
Zhang L.B., Wang E.K.. Metal nanoclusters: new fluorescent probes for sensors and bioimaging[J]. Nano Today,
2014,9:132-157.
doi: 10.1016/j.nantod.2014.02.010
-
[3]
Guo S.J., Wang E.K.. Noble metal nanomaterials: controllable synthesis and application in fuel cells and analytical sensors[J]. Nnao Today,
2011,6:240-264.
doi: 10.1016/j.nantod.2011.04.007
-
[4]
Chen W.Y., Lin J.Y., Chen W.J.. Functional gold nanoclusters as antimicrobial agents for antibiotic-resistant bacteria[J]. Nanomedicine,
2010,5:755-764.
doi: 10.2217/nnm.10.43
-
[5]
Chan P.H., Chen Y.C.. Human serum albumin stabilized gold nanoclusters as selective luminescent probes for Staphylococcus aureus and methicillinresistant Staphylococcus aureus[J]. Anal. Chem.,
2012,84:8952-8956.
doi: 10.1021/ac302417k
-
[6]
Chan P.H., Wong S.Y., Lin S.H., Chen Y.C.. Lysozyme-encapsulated gold nanocluster-based affinity mass spectrometry for pathogenic bacteria[J]. Rapid Commun. Mass Spectrom.,
2013,27:2143-2148.
doi: 10.1002/rcm.v27.19
-
[7]
Chen T.H., Tseng W.L.. (Lysozyme type Ⅵ)-stabilized Au8 clusters: synthesis mechanism and application for sensing of glutathione in a single drop of blood[J]. Small,
2012,8:1912-1919.
doi: 10.1002/smll.201102741
-
[8]
Durgadas C.V., Sharma C.P., Sreenivasan K.. Fluorescent gold clusters as nanosensors for copper ions in live cells[J]. Analyst,
2011,136:933-940.
doi: 10.1039/C0AN00424C
-
[9]
Hu L.Z., Han S., Parveen S.. Highly sensitive fluorescent detection of trypsin based on BSA-stabilized gold nanoclusters[J]. Biosens. Bioelectron.,
2012,32:297-299.
doi: 10.1016/j.bios.2011.12.007
-
[10]
Shang L., Yang L.X., Stockmar F.. Microwave-assisted rapid synthesis of luminescent gold nanoclusters for sensing Hg2+ in living cells using fluorescence imaging[J]. Nanoscale,
2012,4:4155-4160.
doi: 10.1039/c2nr30219e
-
[11]
Li H.C., Guo Y.X., Xiao L.H., Chen B.. Selective and sensitive detection of acetylcholinesterase activity using denatured protein-protected gold nanoclusters as a label-free probe[J]. Analyst,
2014,139:285-289.
doi: 10.1039/C3AN01736B
-
[12]
Wu X., He X.X., Wang K.M.. Ultrasmall near-infrared gold nanoclusters for tumor fluorescence imaging in vivo[J]. Nanoscale,
2010,2:2244-2249.
doi: 10.1039/c0nr00359j
-
[13]
Liang G.H., Ye D.X., Zhang X.X.. One-pot synthesis of Gd3+-functionalized gold nanoclusters for dual model (fluorescence/magnetic resonance) imaging[J]. J. Mater. Chem. B,
2013,1:3545-3552.
doi: 10.1039/c3tb20440e
-
[14]
Qiao J., Mu X.Y., Qi L., Deng J.Q., Mao L.Q.. Folic acid-functionalized fluorescent gold nanoclusters with polymers as linkers for cancer cell imaging[J]. Chem. Commun.,
2013,49:8030-8032.
doi: 10.1039/c3cc44256j
-
[15]
Polavarapu L., Manna M., Xu Q.H.. Biocompatible glutathione capped gold clusters as one-and two-photon excitation fluorescence contrast agents for live cells imaging[J]. Nanoscale,
2011,3:429-434.
doi: 10.1039/C0NR00458H
-
[16]
Li L.L., Liu X., Fu C.H., Tan L.F., Liu H.Y.. Biosynthesis of fluorescent gold nanoclusters for in vitro and in vivo tumor imaging[J]. Opt. Commun.,
2015,355:567-574.
doi: 10.1016/j.optcom.2015.07.023
-
[17]
Chen H.Y., Zhang M., Yang H.B.. Dual fluorescence nano-conjugates based on gold nanoclusters for tumor-targeting imaging[J]. RSC Adv.,
2014,4:8191-8199.
doi: 10.1039/C3RA47453D
-
[18]
Chen H.Y., Li S.L., Li B.W.. Folate-modified gold nanoclusters as nearinfrared fluorescent probes for tumor imaging and therapy[J]. Nanoscale,
2012,4:6050-6064.
doi: 10.1039/c2nr31616a
-
[19]
Vankayala R., Kuo C.L., Nuthalapati K., Chiang C.S., Hwang K.C.. Nucleustargeting gold nanoclusters for simultaneous in vivo fluorescence imaging gene delivery, and NIR-light activated photodynamic therapy[J]. Adv. Funct. Mater.,
2015,25:5934-5945.
doi: 10.1002/adfm.201502650
-
[20]
Zhang X.D., Chen J., Luo Z.T.. Enhanced tumor accumulation of sub-2 nm gold nanoclusters for cancer radiation therapy[J]. Adv. Healthc. Mater.,
2014,3:133-141.
doi: 10.1002/adhm.v3.1
-
[21]
Liu R., Wang Y.L., Yuan Q.. The Au clusters induce tumor cell apoptosis via specifically targeting thioredoxin reductase 1(TrxR1) and suppressing its activity[J]. Chem. Commun.,
2014,50:10687-10690.
doi: 10.1039/C4CC03320E
-
[22]
Chiu W.J., Chen W.Y., Lai H.Z.. Dextran-encapsulated photoluminescent gold nanoclusters: synthesis and application[J]. J. Nanopart. Res.,
2014,162478.
doi: 10.1007/s11051-014-2478-z
-
[23]
Kennedy T.A.C., MacLean J.L., Liu J.W.. Blue emitting gold nanoclusters templated by poly-cytosine DNA at low pH and poly-adenine DNA at neutral pH[J]. Chem. Commun.,
2012,48:6845-6847.
doi: 10.1039/c2cc32841k
-
[24]
Liu G.Y., Shao Y., Ma K.. Synthesis of DNA-templated fluorescent gold nanoclusters[J]. Gold Bull.,
2012,45:69-74.
doi: 10.1007/s13404-012-0049-6
-
[25]
Jiang H., Zhang Y.Y., Wang X.M.. Single cytidine units-templated syntheses of multi-colored water-soluble Au nanoclusters[J]. Nanoscale,
2014,6:10355-10362.
doi: 10.1039/C4NR02180K
-
[26]
Xie J.P., Zheng Y.G., Ying J.Y.. Protein-directed synthesis of highly fluorescent gold nanoclusters[J]. J. Am. Chem. Soc.,
2009,131:888-889.
doi: 10.1021/ja806804u
-
[27]
Kawasaki H., Hamaguchi K., Osaka I., Arakawa R.. pH-Dependent synthesis of pepsin-mediated gold nanoclusters with blue green and red fluorescent emission[J]. Adv. Funct. Mater.,
2011,21:3508-3515.
doi: 10.1002/adfm.201100886
-
[28]
Le Guével X., Daum N., Schneider M.. Synthesis and characterization of human transferrin-stabilized gold nanoclusters[J]. Nanotechnology,
2011,22275103.
doi: 10.1088/0957-4484/22/27/275103
-
[29]
Kawasaki H., Yoshimura K., Hamaguchi K., Arakawa R.. Trypsin-stabilized fluorescent gold nanocluster for sensitive and selective Hg2+ detection[J]. Anal. Sci.,
2011,27:591-596.
doi: 10.2116/analsci.27.591
-
[30]
Retnakumari A., Jayasimhan J., Chandran P.. CD33 monoclonal antibody conjugated Au cluster nano-bioprobe for targeted flow-cytometric detection of acute myeloid leukaemia[J]. Nanotechnology,
2011,22285102.
doi: 10.1088/0957-4484/22/28/285102
-
[31]
Liu C.L., Wu H.T., Hsiao Y.H.. Insulin-directed synthesis of fluorescent gold nanoclusters: preservation of insulin bioactivity and versatility in cell imaging[J]. Angew. Chem.,
2011,50:7056-7060.
doi: 10.1002/anie.v50.31
-
[32]
Kong Y.F., Chen J., Gao F.. Near-infrared fluorescent ribonuclease-Aencapsulated gold nanoclusters: preparation, characterization, cancer targeting and imaging[J]. Nanoscale,
2013,5:1009-1017.
doi: 10.1039/C2NR32760K
-
[33]
Chen Y., Wang Y., Wang C.X.. Papain-directed synthesis of luminescent gold nanoclusters and the sensitive detection of Cu2+[J]. J. Colloid Interface Sci.,
2013,396:63-68.
doi: 10.1016/j.jcis.2013.01.031
-
[34]
Liu P.C., Shang L., Li H.W.. Synthesis of fluorescent a-chymotrypsin Afunctionalized gold nanoclusters and their application to blot-based technology for Hg2+ detection[J]. RSC Adv.,
2014,4:31536-31543.
doi: 10.1039/C4RA05686H
-
[35]
Chattoraj S., Bhattacharyya K.. Fluorescent gold nanocluster inside a live breast cell: etching and higher uptake in cancer cell[J]. J. Phys. Chem. C,
2014,118:22339-22346.
doi: 10.1021/jp506745p
-
[36]
Shao C.Y., Yuan B., Wang H.Q.. Eggshell membrane as a multimodal solid state platform for generating fluorescent metal nanoclusters[J]. J. Mater. Chem.,
2011,21:2863-2866.
doi: 10.1039/c0jm04071a
-
[37]
Wang J.L., Zhang G., Li Q.W.. In vivo self-bio-imaging of tumors through in situ biosynthesized fluorescent gold nanoclusters[J]. Sci. Rep.,
2013,31157.
doi: 10.1038/srep01157
-
[38]
Qian H.F., Zhu M.Z., Wu Z.K., Jin R.C.. Quantum sized gold nanoclusters with atomic precision[J]. Acc. Chem. Res.,
2012,45:1470-1479.
doi: 10.1021/ar200331z
-
[39]
Hu D.H., Sheng Z.H., Zhang P.F.. Hybrid gold-gadolinium nanoclusters for tumor-targeted NIRF/CT/MRI triple-modal imaging in vivo[J]. Nanoscale,
2013,5:1624-1628.
doi: 10.1039/c2nr33543c
-
[40]
Wang Y.L., Xu C., Zhai J.. Label-free Au cluster used for in vivo 2D and 3D computed tomography of murine kidneys[J]. Anal. Chem.,
2015,87:343-345.
doi: 10.1021/ac503887c
-
[41]
Zhang A.L., Tu Y., Qin S.B.. Gold nanoclusters as contrast agents for fluorescent and X-ray dual-modality imaging[J]. J. Colloid Interface Sci.,
2012,372:239-244.
doi: 10.1016/j.jcis.2012.01.005
-
[42]
Chen H.Y., Li B.W., Ren X.Y.. Multifunctional near-infrared-emitting nanoconjugates based on gold clusters for tumor imaging and therapy[J]. Biomaterials,
2012,33:8461-8476.
doi: 10.1016/j.biomaterials.2012.08.034
-
[43]
Zhang X.D., Luo Z.T., Chen J.. Ultrasmall Au10-12(SG)10-12 nanomolecules for high tumor specifi city and cancer radiotherapy[J]. Adv. Mater.,
2014,12:4565-4568.
-
[44]
Rui L., Cao H., Xue Y., Liu L., Xu L., Gao Y., Zhang W.. Functional organic nanoparticles for photodynamic therapy[J]. Chin. Chem. Lett.,
2016,27:1412-1420.
doi: 10.1016/j.cclet.2016.07.011
-
[45]
Gu W., Zhang Q., Zhang T., Li Y., Xiang J., Peng R., Liu J.. Hybrid polymeric nanocapsules loaded with gold nanoclusters and indocyanine green for dual-modal imaging and photothermal therapy[J]. J. Mater. Chem. B,
2016,4:910-919.
doi: 10.1039/C5TB01619C
-
[46]
Khlebtsov B.N., Tuchina E.S., Tuchin V.V., Khlebtsov N.. Multifunctional Au nanoclusters for targeted bioimaging and enhanced photodynamic inactivation of Staphylococcus aureus[J]. RSC Adv.,
2015,5:61639-61649.
doi: 10.1039/C5RA11713E
-
[47]
Quantum P.G.. Fluorescence imaging assisted photodynamic therapy using [hotosensitizer-linked gold quantum clusters[J]. ACS Nano,
2015,9:5825-5832.
doi: 10.1021/acsnano.5b00406
-
[48]
Zhang C., Zhou Z., Zhi X., Ma Y., Wang K., Wang Y., Zhang Y., Fu H.. Insights into the distinguishing stress-induced cytotoxicity of chiral gold nanoclusters and the relationship with GSTP1[J]. Theranostics,
2015,5:134-149.
doi: 10.7150/thno.10363
-
[49]
Kawasaki H., Kumar S., Li G., Zeng C., Kauffman D.R., Yoshimoto J., Iwasaki Y., Jin R.. Generation of singlet oxygen by photoexcited Au25(SR) 18 clusters[J]. Chem. Mater.,
2014,26:2777-2788.
doi: 10.1021/cm500260z
-
[50]
Sakamoto M., Tachikawa T., Fujitsuka M., Majima T.. Photochemical reactivity of gold clusters: dependence on size and spin[J]. Langmuir,
2009,25:13888-13893.
doi: 10.1021/la901552f
-
[51]
Wang L., Li L., Ma H.L., Wang H.. Recent advances in biocompatible supramolecular assemblies for biomolecular detection and delivery[J]. Chin. Chem. Lett.,
2013,24:351-358.
doi: 10.1016/j.cclet.2013.03.018
-
[52]
Rawlings D.E., Dew D., Du Plessis C.. Biomineralization of metal-containing ores and concentrates[J]. Trends Biotechnol.,
2003,21:38-44.
doi: 10.1016/S0167-7799(02)00004-5
-
[53]
Kragh-Hansen U.. Molecular aspects of ligand binding to serum albumin[J]. Pharmacology,
1981,33:17-53.
-
[54]
Wang S., Liu P., Qin Y., Chen Z., Shen J.. Rapid synthesis of protein conjugated gold nanoclusters and their application in tea polyphenol sensing[J]. Sens. Actuators B Chem.,
2016,223:178-185.
doi: 10.1016/j.snb.2015.09.058
-
[55]
Nair L.V., Philips D.S., Jayasree R.S., Ajayaghosh A.. A near-infrared fluorescent nanosensor (AuC@Urease) for the selective detection of blood urea[J]. Small,
2013,9:2673-2677.
doi: 10.1002/smll.v9.16
-
[56]
Wang W., Huang Y., Zhao S., Shao T., Cheng Y.. Human serum albumin (HSA) nanoparticles stabilized with intermolecular disulfide bonds[J]. Chem. Commun.,
2013,49:2234-2236.
doi: 10.1039/c3cc38397k
-
[57]
Sheng Z., Hu D., Zheng M., Zhao P., Liu H., Gao D., Gong P.. Smart human serum albumin-indocyanine green nanoparticles generated by programmed assembly for dual-modal imaging-guided cancer synergistic phototherapy[J]. ACS Nano,
2014,8:12310-12322.
doi: 10.1021/nn5062386
-
[58]
Hui M., Xu X., Li N., An K.. Self-assembly of lysozyme on the surfaces of gold nanoparticles[J]. Chin. Chem. Lett.,
2011,22:973-976.
doi: 10.1016/j.cclet.2011.01.022
-
[59]
Nair L.V., Nair R.V., Jayasree R.S.. An insight into the optical properties of a sub nanosize glutathione stabilized gold cluster[J]. Dalton Trans.,
2016,45:11286-11291.
doi: 10.1039/C6DT01753C

 Login In
Login In






 DownLoad:
DownLoad: