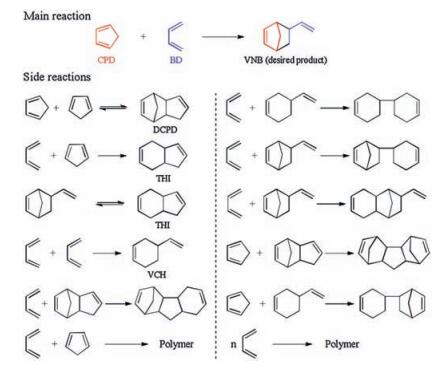
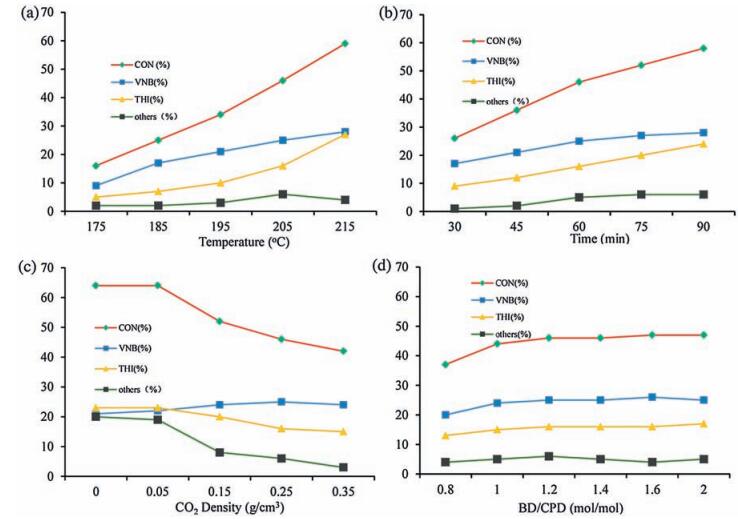
-
[1]
Cong Gao
, Zijian Zhu
, Siwei Li
, Zheng Xi
, Qingqing Sun
, Jie Han
, Rong Guo
. Chiral supramolecular catalysts of helical nanoribbon: More twist, higher enantioselectivity. Chinese Chemical Letters,
2025, 36(3): 109968-.
doi: 10.1016/j.cclet.2024.109968
-
[2]
Yiying Yang
, Dongju Zhang
. Elucidating the Concepts of Thermodynamic Control and Kinetic Control in Chemical Reactions through Theoretical Chemistry Calculations: A Computational Chemistry Experiment on the Diels-Alder Reaction. University Chemistry,
2024, 39(3): 327-335.
doi: 10.3866/PKU.DXHX202309074
-
[3]
Yu-Hang Miao
, Zheng-Xu Zhang
, Xu-Yi Huang
, Yuan-Zhao Hua
, Shi-Kun Jia
, Xiao Xiao
, Min-Can Wang
, Li-Ping Xu
, Guang-Jian Mei
. Catalytic asymmetric dearomative azo-Diels–Alder reaction of 2-vinlyindoles. Chinese Chemical Letters,
2024, 35(4): 108830-.
doi: 10.1016/j.cclet.2023.108830
-
[4]
Ling Fan
, Meili Pang
, Yeyun Zhang
, Yanmei Wang
, Zhenfeng Shang
. Quantum Chemistry Calculation Research on the Diels-Alder Reaction of Anthracene and Maleic Anhydride: Introduction to a Computational Chemistry Experiment. University Chemistry,
2024, 39(4): 133-139.
doi: 10.3866/PKU.DXHX202309024
-
[5]
He Yao
, Wenhao Ji
, Yi Feng
, Chunbo Qian
, Chengguang Yue
, Yue Wang
, Shouying Huang
, Mei-Yan Wang
, Xinbin Ma
. Copper-catalyzed and biphosphine ligand controlled 3,4-boracarboxylation of 1,3-dienes with carbon dioxide. Chinese Chemical Letters,
2025, 36(4): 110076-.
doi: 10.1016/j.cclet.2024.110076
-
[6]
Yue Zhang
, Xiaoya Fan
, Xun He
, Tingyu Yan
, Yongchao Yao
, Dongdong Zheng
, Jingxiang Zhao
, Qinghai Cai
, Qian Liu
, Luming Li
, Wei Chu
, Shengjun Sun
, Xuping Sun
. Ambient electrosynthesis of urea from carbon dioxide and nitrate over Mo2C nanosheet. Chinese Chemical Letters,
2024, 35(8): 109806-.
doi: 10.1016/j.cclet.2024.109806
-
[7]
Daheng Wen
, Weiwei Fang
, Yongmei Liu
, Tao Tu
. Valorization of carbon dioxide with alcohols. Chinese Chemical Letters,
2024, 35(7): 109394-.
doi: 10.1016/j.cclet.2023.109394
-
[8]
Xingxing Jiang
, Yuxin Zhao
, Yan Kong
, Jianju Sun
, Shangzhao Feng
, Xin Lu
, Qi Hu
, Hengpan Yang
, Chuanxin He
. Support effect and confinement effect of porous carbon loaded tin dioxide nanoparticles in high-performance CO2 electroreduction towards formate. Chinese Chemical Letters,
2025, 36(1): 109555-.
doi: 10.1016/j.cclet.2024.109555
-
[9]
Yuan Dong
, Mutian Ma
, Zhenyang Jiao
, Sheng Han
, Likun Xiong
, Zhao Deng
, Yang Peng
. Effect of electrolyte cation-mediated mechanism on electrocatalytic carbon dioxide reduction. Chinese Chemical Letters,
2024, 35(7): 109049-.
doi: 10.1016/j.cclet.2023.109049
-
[10]
Wei-Jia Wang
, Kaihong Chen
. Molecular-based porous polymers with precise sites for photoreduction of carbon dioxide. Chinese Chemical Letters,
2025, 36(1): 109998-.
doi: 10.1016/j.cclet.2024.109998
-
[11]
Yuchen Zhang
, Lifeng Ding
, Zhenghe Xie
, Xin Zhang
, Xiaofeng Sui
, Jian-Rong Li
. Porous sorbents for direct capture of carbon dioxide from ambient air. Chinese Chemical Letters,
2025, 36(3): 109676-.
doi: 10.1016/j.cclet.2024.109676
-
[12]
Zhen Liu
, Zhi-Yuan Ren
, Chen Yang
, Xiangyi Shao
, Li Chen
, Xin Li
. Asymmetric alkenylation reaction of benzoxazinones with diarylethylenes catalyzed by B(C6F5)3/chiral phosphoric acid. Chinese Chemical Letters,
2024, 35(5): 108939-.
doi: 10.1016/j.cclet.2023.108939
-
[13]
You Zhou
, Li-Sheng Wang
, Shuang-Gui Lei
, Bo-Cheng Tang
, Zhi-Cheng Yu
, Xing Li
, Yan-Dong Wu
, Kai-Lu Zheng
, An-Xin Wu
. I2-DMSO mediated tetra-functionalization of enaminones for the construction of novel furo[2′,3′:4,5]pyrimido[1,2-b]indazole skeletons via in situ capture of ketenimine cations. Chinese Chemical Letters,
2025, 36(1): 109799-.
doi: 10.1016/j.cclet.2024.109799
-
[14]
Rong-Nan Yi
, Wei-Min He
. Photocatalytic Minisci-type multicomponent reaction for the synthesis of 1-(halo)alkyl-3-heteroaryl bicyclo[1.1.1]pentanes. Chinese Chemical Letters,
2024, 35(10): 110115-.
doi: 10.1016/j.cclet.2024.110115
-
[15]
Chenhao Zhang
, Qian Zhang
, Yezhou Hu
, Hanyu Hu
, Junhao Yang
, Chang Yang
, Ye Zhu
, Zhengkai Tu
, Deli Wang
. N-doped carbon confined ternary Pt2NiCo intermetallics for efficient oxygen reduction reaction. Chinese Chemical Letters,
2025, 36(3): 110429-.
doi: 10.1016/j.cclet.2024.110429
-
[16]
Jian Yang
, Guang Yang
, Zhijie Chen
. Capturing carbon dioxide from air by using amine-functionalized metal-organic frameworks. Chinese Journal of Structural Chemistry,
2024, 43(5): 100267-100267.
doi: 10.1016/j.cjsc.2024.100267
-
[17]
Xiaxia Xing
, Xiaoyu Chen
, Zhenxu Li
, Xinhua Zhao
, Yingying Tian
, Xiaoyan Lang
, Dachi Yang
. Polyethylene imine functionalized porous carbon framework for selective nitrogen dioxide sensing with smartphone communication. Chinese Chemical Letters,
2024, 35(9): 109230-.
doi: 10.1016/j.cclet.2023.109230
-
[18]
Weidan Meng
, Yanbo Zhou
, Yi Zhou
. Green innovation unleashed: Harnessing tungsten-based nanomaterials for catalyzing solar-driven carbon dioxide conversion. Chinese Chemical Letters,
2025, 36(2): 109961-.
doi: 10.1016/j.cclet.2024.109961
-
[19]
Junjun Huang
, Ran Chen
, Yajian Huang
, Hang Zhang
, Anran Zheng
, Qing Xiao
, Dan Wu
, Ruxia Duan
, Zhi Zhou
, Fei He
, Wei Yi
. Discovery of an enantiopure N-[2-hydroxy-3-phenyl piperazine propyl]-aromatic carboxamide derivative as highly selective α1D/1A-adrenoceptor antagonist and homology modelling. Chinese Chemical Letters,
2024, 35(11): 109594-.
doi: 10.1016/j.cclet.2024.109594
-
[20]
Gangsheng Li
, Xiang Yuan
, Fu Liu
, Zhihua Liu
, Xujie Wang
, Yuanyuan Liu
, Yanmin Chen
, Tingting Wang
, Yanan Yang
, Peicheng Zhang
. Three-step synthesis of flavanostilbenes with a 2-cyclohepten-1-one core by Cu-mediated [5 + 2] cycloaddition/decarboxylation cascade. Chinese Chemical Letters,
2025, 36(2): 109880-.
doi: 10.1016/j.cclet.2024.109880

 Login In
Login In







 DownLoad:
DownLoad: