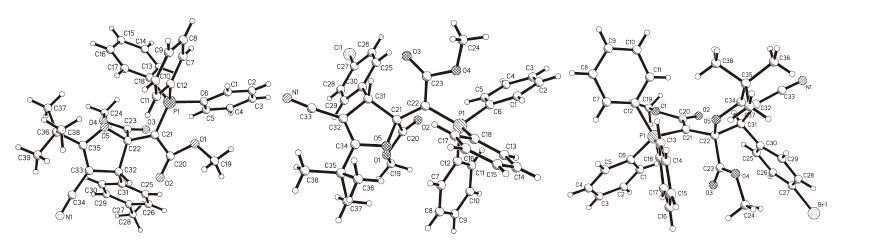
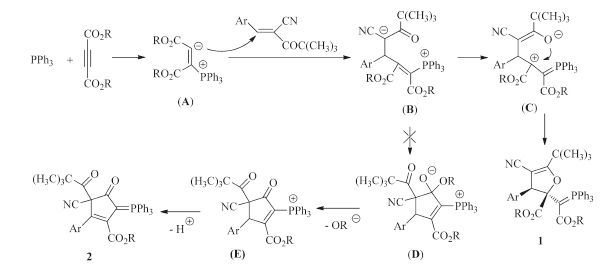
-
[1]
X. Lu, C. Zhang, Z. Xu. Reactions of electron-deficient alkynes and allenes under phosphine catalysis[J]. Acc. Chem. Res.,
2001,34:535-544.
doi: 10.1021/ar000253x
-
[2]
J.L. Methot, W.R. Roush. Nucleophilic phosphine organocatalysis[J]. Adv. Synth. Catal.,
2004,346:1035-1050.
doi: 10.1002/(ISSN)1615-4169
-
[3]
A. Marinetti, A. Voituriez. Enantioselective phosphine organocatalysis[J]. Synlett,
2010:174-195.
-
[4]
Y. Wei, M. Shi. Multifunctional chiral phosphine organocatalysts in catalytic asymmetric Morita-Baylis-Hillman and related reactions[J]. Acc. Chem. Res.,
2010,43:1005-1018.
doi: 10.1021/ar900271g
-
[5]
L.W. Ye, J. Zhou, Y. Tang. Phosphine-triggered synthesis of functionalized cyclic compounds[J]. Chem. Soc. Rev.,
2008,37:1140-1152.
doi: 10.1039/b717758e
-
[6]
B.J. Cowen, S.J. Miller. Enantioselective catalysis and complexity generation from allenoates[J]. Chem. Soc. Rev.,
2009,38:3102-3116.
doi: 10.1039/b816700c
-
[7]
S.L. Xu, Z.J. He. Reactivities of allenoates with aldehydes under the mediation of tertiary phosphines[J]. Sci. Sin. Chim.,
2010,40:856-868.
-
[8]
C. Zhang, X. Lu. Phosphine-catalyzed cycloaddition of 2,3-butadienoates or 2-butynoates with electron-deficient olefins. A novel[3+2] annulation approach to cyclopentenes[J]. J. Org. Chem.,
1995,602906.
doi: 10.1021/jo00114a048
-
[9]
Y.S. Tran, O. Kwon. Phosphine-catalyzed[4+2] annulation:synthesis of cyclohexenes[J]. J. Am. Chem. Soc.,
2007,129:12632-12633.
doi: 10.1021/ja0752181
-
[10]
Q.F. Zhou, F. Yang, Q.X. Guo, S. Xue. Triphenylphosphine-catalyzed isomerization of alkynyl ketones in aqueous solution[J]. Chin. Chem. Lett.,
2007,18:1029-1032.
doi: 10.1016/j.cclet.2007.06.024
-
[11]
W. Wu, X.Y. Zhang, S.X. Kang, Y.M. Gao. Tri(t-butyl)phosphine-assisted selective hydrosilylation of terminal alkynes[J]. Chin. Chem. Lett.,
2010,21:312-316.
doi: 10.1016/j.cclet.2009.11.040
-
[12]
H. Anaraki-Ardakani, M. Noei, A. Tabarzad. Facile synthesis of N-(arylsulfonyl)-4-ethoxy-5-oxo-2,5-dihydro-1H-pyrolle-2,3-dicarboxylates by one-pot three-component reaction[J]. Chin. Chem. Lett.,
2012,23:45-48.
doi: 10.1016/j.cclet.2011.09.010
-
[13]
F. Sheikholeslami-Farahani, Z. Hossaini, F. Rostami-Charati. Solvent-free synthesis of substituted thiopyrans via multicomponent reactions of α-haloketones[J]. Chin. Chem. Lett.,
2014,25:152-154.
doi: 10.1016/j.cclet.2013.10.016
-
[14]
L.Y. Cui, S.H. Guo, B. Li, X.Y. Zhang, X.S. Fan. Synthesis of cyclopentenyl and cyclohexenyl ketones via[3+2] and[4+2] annulations of 1,2-allenic ketones[J]. Chin. Chem. Lett.,
2014,25:55-57.
doi: 10.1016/j.cclet.2013.10.008
-
[15]
H.Y. Duan, J. Ma, Z.Z. Yuan. Phosphine-promoted[3+2] cycloaddition between nonsubstituted MBH carbonates and trifluoromethyl ketones[J]. Chin. Chem. Lett.,
2015,26:646-648.
doi: 10.1016/j.cclet.2015.03.019
-
[16]
X.F. Zhu, C.E. Henry, O. Kwon. Stable tetravalent phosphonium enolate zwitterions[J]. J. Am. Chem. Soc.,
2007,129:6722-6723.
doi: 10.1021/ja071990s
-
[17]
E. Mercier, B. Fonovic, C. Henry, O. Kwon, T. Dudding. Phosphine triggered[3+2] allenoate-acrylate annulation:a mechanistic enlightenment[J]. Tetrahedron Lett.,
2007,48:3617-3620.
doi: 10.1016/j.tetlet.2007.03.030
-
[18]
S. Xu, L. Zhou, R. Ma, H. Song, Z. He. Phosphane-catalyzed[3+2] annulation of allenoates with aldehydes:a simple and efficient synthesis of 2-alkylidenetetrahydrofurans[J]. Chem. Eur. J.,
2009,15:8698-8702.
doi: 10.1002/chem.v15:35
-
[19]
R. Zhou, C.J. Yang, Y.Y. Liu, R.F. Li, Z.J. He. Diastereoselective synthesis of functionalized spirocyclopropyl oxindoles via P(NMe2)3-mediated reductive cyclopropanation[J]. J. Org. Chem.,
2014,79:10709-10715.
doi: 10.1021/jo502106c
-
[20]
Y.Q. Jiang, Y.L. Shi, M. Shi. Chiral phosphine-catalyzed enantioselective construction of γ-butenolides through substitution of Morita-Baylis-Hillman acetates with 2-trimethylsilyloxy furan[J]. J. Am. Chem. Soc.,
2008,130:7202-7203.
doi: 10.1021/ja802422d
-
[21]
S. Xu, L. Zhou, S. Zeng. Phosphine-mediated olefination between aldehydes and allenes:an efficient synthesis of trisubstituted 1,3-dienes with high Eselectivity[J]. Org. Lett.,
2009,11:3498-3501.
doi: 10.1021/ol901334c
-
[22]
Z. Chen, J. Zhang. An unexpected phosphine-catalyzed regio-and diastereoselective[4+1] annulation reaction of modified allylic compounds with activated enones[J]. Chem. Asian J.,
2010,5:1542-1545.
doi: 10.1002/asia.v5:7
-
[23]
P. Xie, Y. Huang, R. Chen. Phosphine-catalyzed domino reaction:highly stereoselective synthesis of trans-2,3-dihydrobenzofurans from salicyl N-thiophosphinyl imines and allylic carbonates[J]. Org. Lett.,
2010,12:3768-3771.
doi: 10.1021/ol101611v
-
[24]
S. Xu, L. Zhou, R. Ma, H. Song, Z. He. Phosphine-mediated stereoselective reductive cyclopropanation of α-substituted allenoates with aromatic aldehydes[J]. Org. Lett.,
2010,12:544-547.
doi: 10.1021/ol902747c
-
[25]
S. Xu, W. Zou, G. Wu, H. Song, Z. He. Stereoselective synthesis of 1,2,3,4-tetrasubstituted dienes from allenoates and aldehydes:an observation of phosphineinduced chemoselectivity[J]. Org. Lett.,
2010,12:3556-3559.
doi: 10.1021/ol101429z
-
[26]
S.C. Chuang, J.C. Deng, F.W. Chan. [3+2] Cycloaddition of dialkyl (E)-Hex-2-en-4-ynedioates to[60] fullerene by phosphane-promoted tandem α(δ')-Michael additions[J]. Eur. J. Org. Chem.,
2012,13:2606-2613.
-
[27]
P.Y. Tseng, S.C. Chuang. Chemo-, regio-and stereoselective tricyclohexylphosphine-catalyzed[3+2] cycloaddition of enynes with[60] fullerene initiated by 1,4-Michael addition:synthesis of cyclopenteno[60] fullerenes and their electrochemical properties[J]. Adv. Synth. Catal.,
2013,355:2165-2171.
doi: 10.1002/adsc.201300255
-
[28]
A.S. Chavan, J.C. Deng, S. C.. Chuang, α(δ')-Michael addition of alkyl amines to dimethyl (E)-hex-2-en-4-ynedioate:synthesis of α,β-dehydroamino acid derivatives[J]. Molecules,
2013,18:2611-2622.
doi: 10.3390/molecules18032611
-
[29]
Y.W. Lin, J.C. Deng, Y.Z. Hsieh, S.C. Chuang. One-pot formation of fluorescent γ-lactams having an α-phosphorus ylide moiety through three-component α(δ')-Michael reactions of phosphines with an enyne and N-tosyl aldimines[J]. Org. Biomol. Chem.,
2014,12:162-170.
doi: 10.1039/C3OB41811A
-
[30]
V. Nair, A.Deepthi , P.B. Beneesh, S. Eringathodi. Anovel three-component reaction of triphenylphosphine, DMAD, and electron-deficient styrenes:facile synthesis of cyclopentenyl phosphoranes[J]. Synthesis,
2006,9:1443-1446.
-
[31]
Y. Han, Y.J. Sheng, C.G. Yan. Convenient synthesis of triphenylphosphanylidene spiro[cyclopent[2] ene-1,3'-indolines] via three-component reactions[J]. Org. Lett.,
2014,16:2654-2657.
doi: 10.1021/ol5008394
-
[32]
G. Hui, J. Sun, C.G. Yan. Synthesis of (triphenylphosphoranylidene) spiro[cyclopentene-1,3'-indole]s by a three-component reaction of triphenylphosphine, dialkyl acetylenedicarboxylates, and 3-(aroylmethylene)-1,3-dihydro-2H-indol-2-ones[J]. Synthesis,
2014,46:2327-2332.
doi: 10.1055/s-00000084
-
[33]
Y. Han, W.J. Qi, Y.J. Sheng, C.G. Yan. Synthesis of triphenylphosphanylidene cyclopent-2-enecaroboxylates with three-component reaction of triphenylphospine, hex-2-en-4-ynedioate, and β-nitrostyrene[J]. Tetrahedron Lett.,
2015,56:5196-5198.
doi: 10.1016/j.tetlet.2015.07.071
-
[34]
J.C. Deng, F.W. Chan, C.W. Kuo. Assembly of dimethyl acetylenedicarboxylate and phosphanes with aldehydes leading to γ-lactones bearing α-phosphorus ylides as Wittig reagents[J]. Eur. J. Org. Chem.,
2012:5738-5747.
-
[35]
J.C. Deng, S.C. Chuang. Three-component and nonclassical reaction of phosphines with enynes and aldehydes:formation of γ-lactones featuring α-phosphorus ylides[J]. Org. Lett.,
2011,13:2248-2251.
doi: 10.1021/ol200527t
-
[36]
Y. Han, L.Y. Guo, C.G. Yan. Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl-2-benzoylcyclopropane-1,1-dicarbonitrile[J]. Heterocycl. Commun.,
2015,21:329-333.

 Login In
Login In






 DownLoad:
DownLoad: