Citation:
Dan-Ting Tan, Xu Shao, Shu-Feng Pang, Yun-Hong Zhang. The effect of CTAB on Na2SO4 nucleation in mixed Na2SO4/CTAB aerosols by FTIR-ATR technology[J]. Chinese Chemical Letters,
;2016, 27(7): 1073-1076.
doi:
10.1016/j.cclet.2016.02.019

-
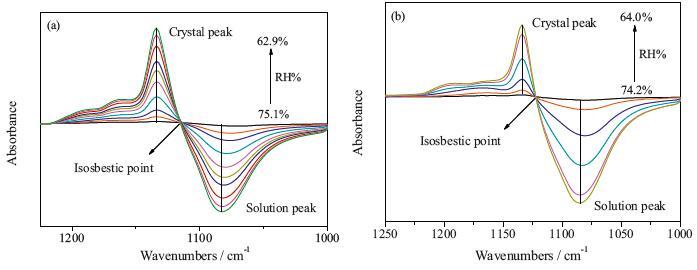
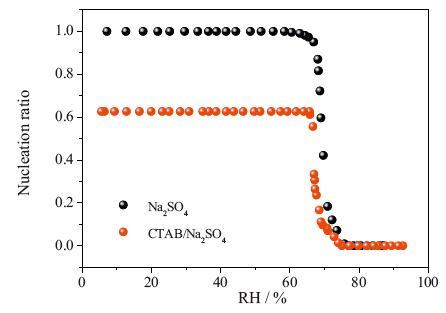
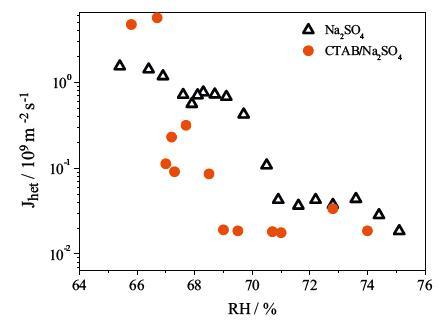
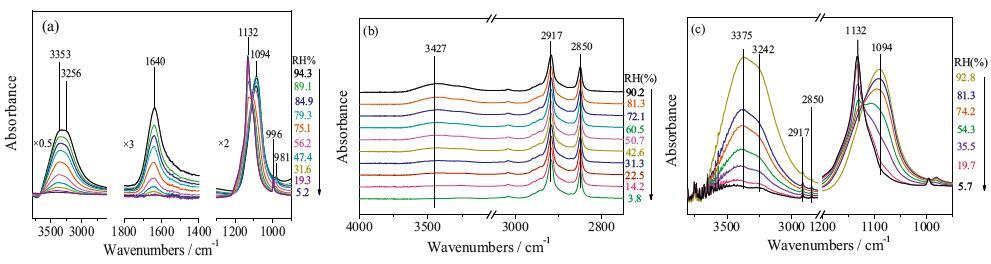
FTIR-ATR technology is used to study the efflorescence kinetic of Na2SO4 and mixed Na2SO4/CTAB aerosols. As the RH decreased linearly, the ν3-SO4-2 band shifts from 1094 cm-1 to 1132 cm-1, suggesting the phase transition of Na2SO4 from solution to crystal phase (Ⅲ). For pure Na2SO4 aerosols, the ERH is 75.1% RH, whereas the efflorescence point ofmixed Na2SO4/CTAB aerosols (74.2%) is lower. By further analysis of IR differential spectra, the ratio of Na2SO4 crystals in mixed aerosols is only 62.7% and the heterogeneous nucleation rate of Na2SO4 in Na2SO4/CTAB mixed aerosols is lower than that in pure Na2SO4 aerosols. They showed that CTAB assembled into reversed micelle and part Na2SO4 droplets are in the core to form core-shell structure, and CTAB shell prevents core Na2SO4 solutions from crystallizing. However, the counter ion Br- for CTAB reversed micelle can interact with Na+ ions, which decreases the crystallization rate of free Na2SO4 droplets and ERH is delayed.
-
Keywords:
- FTIR-ATR,
- Na2SO4/CTAB,
- Efflorescence,
- Nucleation rate,
- RH
-

-
-
[1]
R. Makkonen, A. Asmi, V.M. Kerminen. , Air pollution control and decreasing new particle formation lead to strong climate warming[J]. Atmos. Chem. Phys., 2012,12:1515-1524. doi: 10.5194/acp-12-1515-2012
-
[2]
C.K. Chan, Z.Y. Ha, M.Y. Choi. Study of water activities of aerosols of mixtures of sodium and magnesium salts[J]. Atmos. Environ., 2000,34:4795-4803. doi: 10.1016/S1352-2310(00)00252-1
-
[3]
B. Zuberi, A.K. Bertram, T. Koop, L.T. Molina, M.J. Molina. Heterogeneous freezing of aqueous particles induced by crystallized (NH4)2SO4, Ice, and Letovicite[J]. J. Phys. Chem. A, 2001,105:6458-6464. doi: 10.1021/jp010094e
-
[4]
S.T. Martin. Phase transitions of aqueous atmospheric particles[J]. Chem. Rev., 2000,100:3403-3454. doi: 10.1021/cr990034t
-
[5]
B. Jing, S.R. Tong, Q.F. Liu. , Hygroscopic behavior of multicomponent organic aerosols and their internal mixtures with ammonium sulfate[J]. Atmos. Chem. Phys. Discuss., 2015,15:23357-23405. doi: 10.5194/acpd-15-23357-2015
-
[6]
M. Kajino, M. Aikawa. A model validation study of the washout/rainout contribution of sulfate and nitrate in wet deposition compared with precipitation chemistry data in Japan[J]. Atmos. Environ., 2015,117:124-134. doi: 10.1016/j.atmosenv.2015.06.042
-
[7]
L. Sun, Y. Wang, T.X. Yue. , Evaluation of the behavior of clouds in a region of severe acid rain pollution in southern China: species, complexes, and variations[J]. Environ. Sci. Pollut. Res., 2015,22:14280-14290. doi: 10.1007/s11356-015-4674-5
-
[8]
P.D. Lu, T. He, Y.H. Zhang. Relative humidity anneal effect on hygroscopicity of aerosol particles studied by rapid-scan FTIR-ATR spectroscopy[J]. Geophys. Res. Lett., 2008,35L20812. doi: 10.1029/2008GL035302
-
[9]
Q.N. Zhang, Y. Zhang, C. Cai. , In situ observation on the dynamic process of evaporation and crystallization of sodium nitrate droplets on a ZnSe substrate by FTIR-ATR[J]. J. Phys. Chem. A, 2014,118:2728-2737. doi: 10.1021/jp412073c
-
[10]
J.P. Darr, S.Q. Davis, Y. Kohno, K. McKenna, P. Morales. Morphological effects on the hygroscopic properties of sodium chloride-sodium sulfate aerosols[J]. J. Aerosol Sci., 2014,77:158-167. doi: 10.1016/j.jaerosci.2014.08.002
-
[11]
M. Gysel, E. Weingartner, U. Baltensperger. Hygroscopicity of aerosol particles at low temperatures., 2. Theoretical and experimental hygroscopic properties of laboratory generated aerosols[J]. Environ. Sci. Technol., 2002,36:63-68. doi: 10.1021/es010055g
-
[12]
C. Rodriguez-Navarro, E. Doehne, E. Sebastian. How does sodium sulfate crystallize? Implications for the decay and testing of building materials[J]. Cem. Concr. Res., 2000,30:1527-1534. doi: 10.1016/S0008-8846(00)00381-1
-
[13]
E.R. Gibson, P.K. Hudson, V.H. Grassian. Physicochemical properties of nitrate aerosols: implications for the atmosphere[J]. J. Phys. Chem. A, 2006,110:11785-11799. doi: 10.1021/jp063821k
-
[14]
M. Kanakidou, J.H. Seinfeld, S.N. Pandis. , Organic aerosol and global climate modelling: a review[J]. Atmos. Chem. Phys., 2005,5:1053-1123. doi: 10.5194/acp-5-1053-2005
-
[15]
A.J. Prenni, P.J. DeMott, S.M. Kreidenweis. Water uptake of internally mixed particles containing ammonium sulfate and dicarboxylic acids[J]. Atmos. Environ., 2003,37:4243-4251. doi: 10.1016/S1352-2310(03)00559-4
-
[16]
C. Marcolli, B.P. Luo, T. Peter. Mixing of the organic aerosol fractions: liquids as the thermodynamically stable phases[J]. J. Phys. Chem. A, 2004,108:2216-2224. doi: 10.1021/jp036080l
-
[17]
J.Y. Yu, Y. Zhang, G. Zeng. , Suppression of NaNO3 crystal nucleation by glycerol: micro-raman observation on the efflorescence process of mixed glycerol/NaNO3/water droplets[J]. J. Phys. Chem. B, 2012,116:1642-1650. doi: 10.1021/jp210824e
-
[18]
Q. Zhou, S.F. Pang, Y. Wang, J.B. Ma, Y.H. Zhang. Confocal raman studies of the evolution of the physical state of mixed phthalic acid/ammonium sulfate aerosol droplets and the effect of substrates[J]. J. Phys. Chem. B, 2014,118:6198-6205. doi: 10.1021/jp5004598
-
[19]
T. Koop, H.P. Ng, L.T. Molina, M.J. Molina. A new optical technique to study aerosol phase transitions: the nucleation of ice from H2SO4 aerosols[J]. J. Phys. Chem. A, 1998,102:8924-8931. doi: 10.1021/jp9828078
-
[20]
V.G. Ciobanu, C. Marcolli, U.K. Krieger, A. Zuend, T. Peter. Efflorescence of ammonium sulfate and coated ammonium sulfate particles: evidence for surface nucleation[J]. J. Phys. Chem. A, 2010,114:9486-9495. doi: 10.1021/jp103541w
-
[21]
J.L. Dong, H.S. Xiao, L.J. Zhao, Y.H. Zhang. Spatially resolved Raman investigation on phase separations of mixed Na2SO4/MgSO4 droplets[J]. J. Raman Spectrosc., 2009,40:338-343. doi: 10.1002/jrs.v40:3
-
[22]
H.J. Tong, J.P. Reid, J.L. Dong, Y.H. Zhang. Observation of the crystallization and supersaturation of mixed component NaNO3-Na2SO4 droplets by FTIR-ATR and Raman spectroscopy[J]. J. Phys. Chem. A, 2010,114:12237-12243. doi: 10.1021/jp1080548
-
[23]
Q. Qu, L. Li, W. Bai, C.W. Yan. Initial atmospheric corrosion of zinc in presence of Na2SO4 and (NH4)2SO4[J]. Trans. Nonferrous Met. Soc. China, 2006,16:887-891. doi: 10.1016/S1003-6326(06)60345-2
-
[24]
P.V. Jentzsch, B. Kampe, P. Rö sch, J. Poop. Raman spectroscopic study of crystallization from solutions containing MgSO4 and Na2SO4: Raman spectra of double salts[J]. J. Phys. Chem. A, 2011,115:5540-5546. doi: 10.1021/jp200142n
-
[25]
R.M. Garland, M.E. Wise, M.R. Beaver. , Impact of palmitic acid coating on the water uptake and loss of ammonium sulfate particles[J]. Atmos. Chem. Phys., 2005,5:1951-1961. doi: 10.5194/acp-5-1951-2005
-
[26]
X.N. Feng, H.N. Chen, Y.M. Luan. , In-situ FTIR-ATR spectroscopic observation on the dynamic efflorescence/deliquescence processes of Na2SO4 and mixed Na2SO4/glycerol droplets[J]. Chem. Phys., 2014,430:78-83. doi: 10.1016/j.chemphys.2013.12.009
-
[27]
G. Vali. Freezing rate due to heterogeneous nucleation[J]. J. Atmos. Sci., 1994,51:1843-1856. doi: 10.1175/1520-0469(1994)051<1843:FRDTHN>2.0.CO;2
-
[1]
-

-
-
[1]
Ying Zhao , Yin-Hang Chai , Tian Chen , Jie Zheng , Ting-Ting Li , Francisco Aznarez , Li-Long Dang , Lu-Fang Ma . Size-controlled synthesis and near-infrared photothermal response of Cp* Rh-based metalla[2]catenanes and rectangular metallamacrocycles. Chinese Chemical Letters, 2024, 35(6): 109298-. doi: 10.1016/j.cclet.2023.109298
-
[2]
Peiyan Zhu , Yanyan Yang , Hui Li , Jinhua Wang , Shiqing Li . Rh(Ⅲ)‐Catalyzed sequential ring‐retentive/‐opening [4 + 2] annulations of 2H‐imidazoles towards full‐color emissive imidazo[5,1‐a]isoquinolinium salts and AIE‐active non‐symmetric 1,1′‐biisoquinolines. Chinese Chemical Letters, 2024, 35(10): 109533-. doi: 10.1016/j.cclet.2024.109533
-
[3]
Bin Feng , Tao Long , Ruotong Li , Yuan-Li Ding . Rationally constructing metallic Sn-ZnO heterostructure via in-situ Mn doping for high-rate Na-ion batteries. Chinese Chemical Letters, 2025, 36(2): 110273-. doi: 10.1016/j.cclet.2024.110273
-
[4]
Cuiwu MO , Gangmin ZHANG , Chao WU , Zhipeng HUANG , Chi ZHANG . A(NH2SO3) (A=Li, Na): Two ultraviolet transparent sulfamates exhibiting second harmonic generation response. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1387-1396. doi: 10.11862/CJIC.20240045
-
[5]
Tao Long , Peng Chen , Bin Feng , Caili Yang , Kairong Wang , Yulei Wang , Can Chen , Yaping Wang , Ruotong Li , Meng Wu , Minhuan Lan , Wei Kong Pang , Jian-Fang Wu , Yuan-Li Ding . Reinforced concrete-like Na3.5V1.5Mn0.5(PO4)3@graphene hybrids with hierarchical porosity as durable and high-rate sodium-ion battery cathode. Chinese Chemical Letters, 2024, 35(4): 109267-. doi: 10.1016/j.cclet.2023.109267
-
[6]
Chao Chen , Wenwen Yu , Guangen Huang , Xuelian Ren , Xiangli Chen , Yixin Li , Shenggui Liang , Mengmeng Xu , Mingyue Zheng , Yaxi Yang , He Huang , Wei Tang , Bing Zhou . Asymmetric macrocyclization enabled by Rh(Ⅲ)-catalyzed CH activation: Enantioenriched macrocyclic inhibitor of Zika virus infection. Chinese Chemical Letters, 2024, 35(11): 109574-. doi: 10.1016/j.cclet.2024.109574
-
[7]
Luyun Zhang , Ding Liu , Huri Piao , Zhenhua Jia , Fen-Er Chen . A modified Bis-OPNN phosphorus ligand for Rh-catalyzed linear-selective hydroformylation of alkenes. Chinese Chemical Letters, 2025, 36(7): 110640-. doi: 10.1016/j.cclet.2024.110640
-
[8]
Xiaojun Wang , Yizhou Zhang , Linwei Guo , Jianwei Li , Peng Wang , Lei Yang , Zhiming Liu . V2CTX MXene-derived ammonium vanadate with robust carbon skeleton for superior rate aqueous zinc-ion batteries. Chinese Chemical Letters, 2025, 36(8): 111231-. doi: 10.1016/j.cclet.2025.111231
-
[9]
Fanjun Kong , Jing Zhang , Yuting Tang , Chencheng Sun , Chunfu Lin , Tao Zhang , Wangsheng Chu , Li Song , Liang Zhang , Shi Tao . Introducing high-valence element into P2-type layered cathode material for high-rate sodium-ion batteries. Chinese Chemical Letters, 2025, 36(8): 110993-. doi: 10.1016/j.cclet.2025.110993
-
[10]
Shulei Hu , Yu Zhang , Xiong Xie , Luhan Li , Kaixian Chen , Hong Liu , Jiang Wang . Rh(Ⅲ)-catalyzed late-stage C-H alkenylation and macrolactamization for the synthesis of cyclic peptides with unique Trp(C7)-alkene crosslinks. Chinese Chemical Letters, 2024, 35(8): 109408-. doi: 10.1016/j.cclet.2023.109408
-
[11]
Xinghui Yao , Zhouyu Wang , Da-Gang Yu . Sustainable electrosynthesis: Enantioselective electrochemical Rh(III)/chiral carboxylic acid-catalyzed oxidative CH cyclization coupled with hydrogen evolution reaction. Chinese Chemical Letters, 2024, 35(9): 109916-. doi: 10.1016/j.cclet.2024.109916
-
[12]
Jianing He , Xiao Wang , Zijian Wang , Ruize Jiang , Ke Wang , Rui Zhang , Huilin Wang , Baokang Geng , Hongyi Gao , Shuyan Song , Hongjie Zhang . Investigation on Cu promotion effect on Ce-based solid solution-anchored Rh single atoms for three-way catalysis. Chinese Chemical Letters, 2025, 36(2): 109640-. doi: 10.1016/j.cclet.2024.109640
-
[13]
Chong Tang , Zhong Qiu , Chen Li , Tengfei Zhang , Renhong Chen , Yifa Sheng , Xinhui Xia , Yongqi Zhang , Jun Liu . Rational synthesis of vertical graphene supported TiN@N-Li4Ti5O12 as advanced high-rate electrodes for lithium-ion batteries. Chinese Chemical Letters, 2025, 36(11): 110589-. doi: 10.1016/j.cclet.2024.110589
-
[14]
Guangchang Yang , Shenglong Yang , Jinlian Yu , Yishun Xie , Chunlei Tan , Feiyan Lai , Qianqian Jin , Hongqiang Wang , Xiaohui Zhang . Regulating local chemical environment in O3-type layered sodium oxides by dual-site Mg2+/B3+ substitution achieves durable and high-rate cathode. Chinese Chemical Letters, 2024, 35(9): 109722-. doi: 10.1016/j.cclet.2024.109722
-
[15]
Shuangxi Li , Huijun Yu , Tianwei Lan , Liyi Shi , Danhong Cheng , Lupeng Han , Dengsong Zhang . NOx reduction against alkali poisoning over Ce(SO4)2-V2O5/TiO2 catalysts by constructing the Ce4+–SO42− pair sites. Chinese Chemical Letters, 2024, 35(5): 108240-. doi: 10.1016/j.cclet.2023.108240
-
[16]
Yaping Wang , Pengcheng Yuan , Zeyuan Xu , Xiong-Xiong Liu , Shengfa Feng , Mufan Cao , Chen Cao , Xiaoqiang Wang , Long Pan , Zheng-Ming Sun . Ti3C2Tx MXene in-situ transformed Li2TiO3 interface layer enabling 4.5 V-LiCoO2/sulfide all-solid-state lithium batteries with superior rate capability and cyclability. Chinese Chemical Letters, 2024, 35(6): 108776-. doi: 10.1016/j.cclet.2023.108776
-
[17]
Yanqiu Xu , Xuanli Chen , Yin Li , Keyu Zhang , Shaoze Zhang , Junxian Hu , Yaochun Yao . Progress in Na2FePO4F cathodes for energy storage: Fabrication, modification and application. Chinese Chemical Letters, 2025, 36(12): 110574-. doi: 10.1016/j.cclet.2024.110574
-
[18]
Hailian Tang , Siyuan Chen , Qiaoyun Liu , Guoyi Bai , Botao Qiao , Liu Fei . Stabilized Rh/hydroxyapatite Catalyst for Furfuryl Alcohol Hydrogenation: Application of Oxidative Strong Metal-Support Interactions in Reducing Conditions. Acta Physico-Chimica Sinica, 2025, 41(4): 100036-0. doi: 10.3866/PKU.WHXB202408004
-
[19]
Ruofan Yin , Zhaoxin Guo , Rui Liu , Xian-Sen Tao . Ultrafast synthesis of Na3V2(PO4)3 cathode for high performance sodium-ion batteries. Chinese Chemical Letters, 2025, 36(2): 109643-. doi: 10.1016/j.cclet.2024.109643
-
[20]
Débora Ferreira dos Santos Morais , José Luis Tirado , Carlos Pérez-Vicente , Fabiana Villela da Motta , Pedro Lavela , Mauricio Bomio , Sergio Lavela . Unlocking the performance of sodium-ion batteries by coating Na3V2(PO4)3 with Nb2O5. Acta Physico-Chimica Sinica, 2026, 42(2): 100180-0. doi: 10.1016/j.actphy.2025.100180
-
[1]
Metrics
- PDF Downloads(1)
- Abstract views(1302)
- HTML views(44)

 Login In
Login In




 DownLoad:
DownLoad: