 图1
生物活性异喹啉酮类化合物及目标化合物3
Figure1.
Biologically activities of isoquinolinone compounds and the target compounds 3
图1
生物活性异喹啉酮类化合物及目标化合物3
Figure1.
Biologically activities of isoquinolinone compounds and the target compounds 3

Citation: Wang Baoqu, Kong Lingbin, Luo Qin, Lin Jun, Yan Shengjiao. One-Step Synthesis of Isoquinolinone Compounds[J]. Chinese Journal of Organic Chemistry, 2017, 37(11): 2972-2977. doi: 10.6023/cjoc201705038

一步法合成异喹啉酮类化合物
English
One-Step Synthesis of Isoquinolinone Compounds
-
Key words:
- environment friendly
- / atom economy
- / heterocyclic ketone aminals
- / isoquinolinones
-
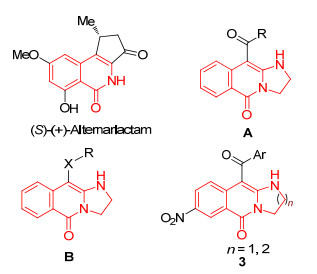
异喹啉酮类化合物广泛存在于自然界中, 许多药物或药物中间体中均含有异喹啉酮结构单元.如对癌细胞、真菌具有细胞毒素作用的天然产物(S)-(+)-Alternarlactam(图 1)[1], narciclasine, lycoricidine等[2].异喹啉酮类化合物具有抗疟疾(图 1, A)[3]、杀虫(图 1, B)[4]、抗微生物[5]、抗肿瘤[6]、抗抑郁[7]、抗菌[8]、镇痛消炎[9]等生物活性[10].由于其重要的生物及药理活性, 异喹啉酮多年来一直被化学及药物学家们广泛关注.该类化合物的合成一直是有机合成的热点之一, 其合成方法有经典的付克反应、酸与胺的酰胺化反应、Diels-Alder反应、金属催化的偶联反应等[11].此外, 近年来为实现异喹啉酮类化合物的合成, Robison课题组[12]采用喹啉化合物在酸性条件下重排反应; Georgian等[13a]利用异喹啉磺酸加成氧化; 肖文精课题组[13b]用自由基参与的分子内氢胺化反应; Hudlicky课题组[14]用环己基氨基甲酸酯类化合物经关环反应(Lycorididine).但这些方法通常反应条件较苛刻, 反应时间长, 收率较低.所以发展简洁、高效合成异喹啉酮类化合物的方法对筛选、发现其生物活性及进一步研究开发药物具有重要意义.
 图1
生物活性异喹啉酮类化合物及目标化合物3
Figure1.
Biologically activities of isoquinolinone compounds and the target compounds 3
图1
生物活性异喹啉酮类化合物及目标化合物3
Figure1.
Biologically activities of isoquinolinone compounds and the target compounds 3
杂环烯酮缩胺(Heterocyclic ketone aminals)[15~19]是一类重要多功能合成子, 其具有两个给电子的氨基, 一个吸电子的羰基和高度极化的碳碳双键[20].由于C(3)的电子云密度比N(1)和N(5)的大, 所以杂环烯酮缩胺的烷基或芳基化反应通常优先发生在C(3)上[21].我们利用杂环烯酮缩胺的C(3)和N(1)为双亲核位点与双亲电试剂反应区域选择性反应合成异喹啉酮类化合物(Eq. 1).此外我们以硝基取代的邻氟苯甲酸酯为原料目的是为了在目标化合物中引入硝基, 便于今后将硝基进行衍生化, 如合成酰胺基取代、脲桥结构等的异喹啉酮类化合物.
目标产物的合成用硝基取代的邻氟苯甲酸甲酯1与杂环烯酮缩胺(HKAs, 2)反应, 通过杂环烯酮缩胺(HKAs) α-C选择性对硝基取代的邻氟苯甲酸甲酯的氟原子进行亲核取代反应, 然后硝基取代的邻氟苯甲酸甲酯的酯基对杂环烯酮缩胺(HKAs)胺基进行酰化反应, 从而合成异喹啉酮类化合物(Eq. 2).本方法符合绿色合成原则, 操作简单, 反应条件温和, 反应时间短, 原子利用率高, 收率高.
1 结果与讨论
1.1 反应条件的筛选
称取1.1 mmol的2-氟-5-硝基苯甲酸甲酯(1a)和1.0 mmol的杂环烯酮缩胺(2c), 将他们加入到25 mL圆底烧瓶中, 加入15 mL溶剂搅拌, 之后对反应溶剂、催化剂、时间以及温度对收率的影响进行了筛选, 结果见表 1.

Entry Solvent Catalyst T/℃ Time/h Yieldb/% 1 EtOH — r.t. 12 — 2 DCM — r.t. 12 — 3 EtOH — Reflux 12 — 4 DMF — Reflux 12 — 5 Toluene — Reflux 12 20 6 1, 4-Dioxane — Reflux 12 40 7 CH3CN — Reflux 12 — 8c 1, 4-Dioxane K2CO3 Reflux 12 30 9c 1, 4-Dioxane Et3N Reflux 12 — 10c 1, 4-Dioxane Piperidine Reflux 12 — 11c 1, 4-Dioxane Cs2CO3 Reflux 12 91 12c 1, 4-Dioxane Cs2CO3 Reflux 8 70 13c 1, 4-Dioxane Cs2CO3 Reflux 20 90 14d 1, 4-Dioxane Cs2CO3 Reflux 12 92 15e 1, 4-Dioxane Cs2CO3 Reflux 12 90 aReactions were carried out using 1a (1.1 mmol), 2c (1.0 mmol), and solvent (15 mL). bIsolated yield based on HKA 2c.c Catalyst 1.0 mmol. d Catalyst 0.05 mmol. e Catalyst 0.1 mmol. 最初尝试了在室温下, 分别以乙醇(EtOH)和N, N-二甲基甲酰胺(DMF)为溶剂, 无催化剂条件下反应, 以薄层色谱(TLC)作为检测手段, 结果显示(表 1, Entries 1, 2), 两种情况下都无目标化合物3c生成.然后我们考察了不同溶剂中无催化剂条件下, 加热回流考察是否有目标化合物生成.结果显示甲苯、1, 4-二氧六环(表 1, Entries 3~7)作溶剂时都能得到目标产物, 但是1, 4-二氧六环作溶剂时收率为40%, 表明1, 4-二氧六环是最优溶剂.在此基础上, 进一步研究了催化剂对本反应的影响, 结果显示K2CO3和Cs2CO3作为催化剂时反应能进行, 而Cs2CO3作为催化剂时收率最高, 达到91%(表 1, Entry 11).进一步考虑反应时间对收率的影响, 结果显示, 缩短反应时间为8 h时, 收率降低为70%.如果将反应时间延长至20 h, 收率与反应12 h相当, 收率不再增加(表 1, Entries 11 vs. 13).因此最佳反应时间为12 h.在此基础上, 进一步考察催化剂用量对反应收率的影响, 我们将催化剂Cs2CO3用量调整为原料2c用量的5 mol%时, 反应以92%收率获得目标化合物3c.将Cs2CO3用量调整为原料2c用量的10 mol%时, 反应收率为90%(表 1, Entries 14 vs 15).因此5 mol%的催化剂对该反应已经足够了.综合来看, 最优的合成条件为以1, 4-二氧六环作为溶剂, 底物2-氟-5-硝基苯甲酸甲酯/杂环烯酮缩胺物质的量之比为1.1:1, 5 mol%的Cs2CO3催化下回流12 h.反应结束后反应液冷却到室温, 柱分离干燥即可以92%的收率得目标化合物3c.
1.2 反应底物对收率影响
在最佳条件基础上, 为进一步地验证反应的普适性, 选择以2-氟-5-硝基苯甲酸甲酯(1a)与杂环烯酮缩胺芳基上吸电子的氟、氯和供电子的甲基、甲氧基2a~2j反应, 成功地合成了10个异喹啉酮类化合物3a~3j, 结果见表 2.实验结果表明, 杂环烯酮缩胺芳环为吸电子基团(F, Cl)时比芳环为供电子基团(Me, OMe)能获得更高的收率(表 2, Entries 1~5);同时, 杂环烯酮缩胺芳基上供电子基团(Me、OMe)取代时, 产物收率无明显变化(表 2, Entries 3~5), 而在杂环烯酮缩胺为六元环时比五元环收率有所下降(表 2, Entries 6~10), 但均能达到90%以上的收率.而当用2-氟-4-硝基苯甲酸甲酯(1b)作反应底物与杂环烯酮缩胺2a~2b反应时, 反应以40%~43%收率得目标化合物3k~3l, 这可能是由于1b中的氟原子处在强吸电子硝基的间位而非对位, 这造成化合物1b中与氟原子相连的碳原子的电正性没有化合物1a高.导致杂环烯酮缩胺(HKAs) α-C选择性对1b的氟原子进行亲核取代反应较1a难, 收率有所降低.

Entry 1 n Ar 3 Yieldb/% 1 1a 1 4-Fluorophenyl 3a 98 2 1a 1 4-Chlorophenyl 3b 92 3 1a 1 Phenyl 3c 92 4 1a 1 4-Methylphenyl 3d 90 5 1a 1 4-Methoxyphenyl 3e 90 6 1a 2 4-Fluorophenyl 3f 95 7 1a 2 4-Chlorophenyl 3g 93 8 1a 2 Phenyl 3h 90 9 1a 2 4-Methylphenyl 3i 92 10 1a 2 4-Methoxyphenyl 3j 90 11 1b 2 4-Fluorophenyl 3k 43 12 1b 2 4-Chlorophenyl 3l 40 aAll reactions were run under the following conditions: 1 (1.1 mmol) and 2 (1.0 mmol) were refluxed in the solvent of 1, 4-dioxane (15 mL) for 12 h. bIsolated yield based on HKAs (2). 1.3 反应机理
该反应可能的机理如Scheme 1. HKAs (2) α-C对硝基取代的邻氟苯甲酸甲酯1进行亲核加成反应得中间体4, 中间体4脱去F-形成化合物5.中间体5经过亚胺-烯胺互变形成中间体6, 化合物6经分子内酰化反应脱去一分子甲醇得到目标产物3.
2 结论
成功建立了一种绿色、简洁、高效合成具有潜在生物活性的异喹啉酮类化合物的方法.该方法以硝基取代的邻氟苯甲酸甲酯1与杂环烯酮缩胺类化合物2在1, 4-二氧六环溶剂中, 碳酸铯为碱的条件下加热回流反应, 通过亲核加成、消除、酰化反应快速构建结构新颖的异喹啉酮类化合物3.该类硝基取代的异喹啉酮类化合物的一锅法制备为便于今后进行结构修饰和衍生化, 同时该类化合物的合成为今后进行抗肿瘤活性研究打下基础.该方法不仅具有合成路线简单、原料易得等优点, 更重要的是为杂环烯酮缩胺甚至烯胺类化合物为砌块合成具有潜在生物活性的类天然杂环化合物库提供一种十分有效的合成方法.
3 实验部分
3.1 仪器和试剂
控温型电磁搅拌器; 傅里叶红外光谱仪(Thermo Nicolet Avatar 360型); 高分辨质谱仪(Agilent CL/Msd TOF); 核磁共振仪Bruck DRX500 (1H: 500 MHz, 13C: 125 MHz), Bruck DRX600 (1H: 600 MHz, 13C: 150 MHz); 熔点仪(XT-4A控温型显微熔点测定仪). GF254高效薄层层析板, 分析纯或化学纯试剂, HKAs (2)按文献[22]制备.
3.2 实验方法
2.3.1 化合物3的合成
称取1.1 mmol硝基取代的邻氟苯甲酸甲酯1和1.0 mmol杂环烯酮缩胺2加入到25 mL圆底烧瓶中, 加入15 mL 1, 4-二氧六环和碳酸铯(0.05 mmol, 16 mg), 充分搅拌并加热回流12 h, 薄层色谱(TLC)监测直至原料消失, 停止反应.待反应液冷却至室温后过滤, 柱分离[V(石油醚):V(乙酸乙酯)=1:2]后在红外灯下干燥, 得到目标产物3a~3l, 收率为40%~98%.所有的化合物都经过核磁共振、红外、高分辨质谱验证.
10-(4-氟苯甲酰基)-7-硝基-2, 3-二氢咪唑并[1, 2-b]异喹啉-5(1H)-酮(3a):黄色固体, m.p. 288~290 ℃; Rf=0.3 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (500 MHz, DMSO-d6) δ: 8.89 (s, 1H, NH), 8.74 (s, 1H, ArH), 7.98 (d, J=9.5 Hz, 1H, ArH), 7.65 (t, J=6.5 Hz, 2H, ArH), 7.28 (t, J=8.5 Hz, 2H, ArH), 6.97 (d, J=9.5 Hz, 1H, ArH), 4.20 (t, J=9.0 Hz, 2H, CH2), 3.82 (t, J=9.0 Hz, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 190.8, 159.6, 155.4, 143.7, 141.7, 131.8 (d, J=8.8 Hz), 125.9, 125.3, 123.5, 119.6, 92.5, 43.8, 43.4; IR (KBr) ν: 3358, 1671, 1606, 1571, 1494, 1321, 1244 cm-1; HRMS (TOF ES+) calcd for C18H13FN3O4[M+H]+ 354.0890, found 354.0883.
10-(4-氯苯甲酰基)-7-硝基-2, 3-二氢咪唑并[1, 2-b]异喹啉-5(1H)-酮(3b):黄色固体. m.p. 275~277 ℃; Rf=0.3 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (500 MHz, DMSO-d6) δ: 8.97 (s, 1H, NH), 8.73 (s, 1H, ArH), 8.00 (d, J=8.8 Hz, 1H, ArH), 7.59 (d, J=8.0 Hz, 2H, ArH), 7.52 (d, J=8.0 Hz, 2H, ArH), 6.97 (d, J=9.2 Hz, 1H, ArH), 4.20 (t, J=8.3 Hz, 2H, CH2), 3.82 (t, J=8.8 Hz, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 193.6, 161.2, 155.6, 143.5, 142.3, 141.6, 132.4, 129.6, 129.5, 126.3, 125.5, 124.6, 117.8, 93.8, 41.2, 41.0; IR (KBr) ν: 3416, 1669, 1618, 1568, 1488, 1323, 1249 cm-1; HRMS (TOF ES+) calcd for C18H13ClN3O4 [M+H]+ 370.0595, found 370.0590.
10-苯甲酰基-7-硝基-2, 3-二氢咪唑并[1, 2-b]异喹啉-5(1H)-酮(3c): 黄色固体, m.p. 270~272 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.99 (s, 1H, NH), 8.66 (s, 1H, ArH), 7.87 (d, J=8.5 Hz, 1H, ArH), 7.51~7.56 (m, 3H, ArH), 7.44~7.47 (m, 2H, ArH), 6.82 (d, J=9.2 Hz, 1H, ArH), 4.19 (t, J=8.9 Hz, 2H, CH2), 3.83 (t, J=8.5 Hz, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 192.6, 159.8, 155.8, 144.0, 141.9, 141.5, 132.6, 129.6, 129.2, 126.0, 123.8, 119.8, 93.0, 44.2, 43.8; IR (KBr) ν: 3302, 1682, 1616, 1566, 1494, 1319, 1247 cm-1; HRMS (TOF ES+) calcd for C18H14N3O4 [M+H]+ 336.0984, found 336.0983.
10-(4-甲基苯甲酰基)-7-硝基-2, 3-二氢咪唑并[1, 2-b]异喹啉-5(1H)-酮(3d):黄色固体. m.p. 269~271 ℃; Rf=0.3 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (500 MHz, DMSO-d6) δ: 8.85 (s, 1H, NH), 8.74 (s, 1H, ArH), 8.00 (d, J=8.8 Hz, 1H, ArH), 7.49 (d, J=8.0 Hz, 2H, ArH), 7.26 (d, J=7.5 Hz, 2H, ArH), 6.97 (d, J=9.0 Hz, 1H, ArH), 4.20 (d, J=9.0 Hz, 2H, CH2), 3.83 (d, J=9.5 Hz, 2H, CH2), 2.38 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) δ: 190.8, 165.6, 163.6, 159.5, 155.3, 143.6, 141.6, 137.4 (d, J=3.8 Hz), 131.7 (d, J=8.8 Hz), 125.8, 125.2, 123.4, 119.5, 116.1 (d, J=21.3 Hz), 92.5, 43.8, 43.4, 39.5; IR (KBr) ν: 3455, 1674, 1621, 1563, 1479, 1316, 1249 cm-1; HRMS (TOF ES+) calcd for C19H16N3O4 [M+H]+ 350.1141, found 350.1137.
10-(4-甲氧基苯甲酰基)-7-硝基-2, 3-二氢咪唑并[1, 2-b]异喹啉-5(1H)-酮(3e):黄色固体, m.p. 268~270 ℃; Rf=0.3 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (500 MHz, DMSO-d6) δ: 8.70 (s, 1H, NH), 8.67 (s, 1H, ArH), 8.00 (d, J=8.8 Hz, 1H, ArH), 7.58 (d, J=8.0 Hz, 2H, ArH), 6.98 (t, J=9.0 Hz, 3H, ArH), 4.19 (t, J=9.0 Hz, 2H, CH2), 3.83 (s, 2H, CH2), 3.80 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) δ: 191.2, 162.9, 159.5, 154.9, 143.9, 141.4, 133.0, 131.4, 125.8, 125.1, 123.5, 119.2, 114.4, 92.6, 55.9, 43.9, 43.3; IR (KBr) ν: 3445, 1676, 1625, 1608, 1495, 1328, 1264 cm-1; HRMS (TOF ES+) calcd for C19H16N3O5 [M+H]+ 366.1090, found 366.1086.
11-(4-氟苯甲酰基)-8-硝基-3, 4-二氢-1H-嘧啶并[1, 2-b]异喹啉-6(2H)-酮(3f):黄色固体, m.p. 236~238 ℃; Rf=0.3 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (500 MHz, DMSO-d6) δ: 8.73 (s, 1H, NH), 7.87 (d, J=9.5 Hz, 1H, ArH), 7.60 (t, J=6.0 Hz, 2H, ArH), 7.24 (t, J=8.5 Hz, 2H, ArH), 6.79 (d, J=9.0 Hz, 1H, ArH), 4.07 (t, J=5.0 Hz, 2H, CH2), 3.49 (s, 2H, CH2), 2.06 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 191.8, 163.5(d, J=248.8 Hz), 160.9, 153.1, 143.1, 141.3, 138.3, 132.0 (d, J=8.8 Hz), 125.9, 125.4, 124.2, 117.5, 116.3, 116.1, 93.4, 40.9, 39.2, 19.1; IR (KBr) ν: 3444, 1674, 1600, 1566, 1496, 1329, 1256 cm-1; HRMS (TOF ES+) calcd for C19H15FN3O4 [M+H]+ 368.1047, found 368.1041.
11-(4-氯苯甲酰基)-8-硝基-3, 4-二氢-1H-嘧啶并[1, 2-b]异喹啉-6(2H)-酮(3g):黄色固体, m.p. 235~237 ℃; Rf=0.3 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (500 MHz, DMSO-d6) δ: 10.4 (s, 1H, NH), 8.7 (d, J=2.5 Hz, 1H, ArH), 7.87 (q, J=3.0 Hz, 1H, ArH), 7.54 (d, J=8.5 Hz, 2H, ArH), 7.47 (d, J=8.5 Hz, 2H, ArH), 6.78 (d, J=9.0 Hz, 1H, ArH), 4.06 (t, J=5.5 Hz, 2H, CH2), 3.50 (s, 2H, CH2), 2.07 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 191.6, 160.9, 153.2, 143.0, 141.4, 140.6, 136.7, 131.1, 129.3, 126.0, 125.4, 124.2, 117.6, 93.3, 40.8, 39.2, 19.0; IR (KBr) ν: 3456, 1675, 1603, 1577, 1484, 1319, 1273 cm-1; HRMS (TOF ES+) calcd for C19H15ClN3O4 [M+H]+ 384.0751, found 384.0745.
11-苯甲酰基-8-硝基-3, 4-二氢-1H-嘧啶并[1, 2-b]异喹啉-6(2H)-酮(3h):黄色固体, m.p. 251~253 ℃; Rf=0.3 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (500 MHz, DMSO-d6) δ: 10.47 (s, 1H, NH), 8.68 (d, J=2.6 Hz, 1H, ArH), 7.77 (q, J=2.6 Hz, 1H, ArH), 7.51 (t, J=10.5 Hz, 3H, ArH), 7.36~7.43 (m, 2H, ArH), 6.71 (d, J=9.3 Hz, 1H, ArH), 4.06 (t, J=5.8 Hz, 2H, CH2), 3.56 (s, 2H, CH2), 2.06 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 193.6, 161.2, 153.6, 143.5, 142.3, 141.6, 132.4, 129.6, 129.5, 126.3, 125.5, 124.6, 117.8, 93.8, 41.2, 39.6, 19.5; IR (KBr) ν: 3417, 1669, 1603, 1561, 1495, 1324, 1257 cm-1; HRMS (TOF ES+) calcd for C19H16N3O4 [M+H]+ 350.1141, found 350.1137.
11-(4-甲基苯甲酰基)-8-硝基-3, 4-二氢-1H-嘧啶并[1, 2-b]异喹啉-6(2H)-酮(3i):黄色固体, m.p. 248~250 ℃; Rf=0.3 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (500 MHz, DMSO-d6) δ: 10.26 (s, 1H, NH), 8.7 (d, J=8.5 Hz, 1H, ArH), 7.82 (d, J=9.0 Hz, 1H, ArH), 7.44 (d, J=8.0 Hz, 2H, ArH), 7.22 (d, J=7.5 Hz, 2H, ArH), 6.77 (t, J=10.5 Hz, 1H, ArH), 4.07 (t, J=5.0 Hz, 2H, CH2), 3.48 (s, 2H, CH2), 2.33 (d, J=10.5 Hz, 3H, CH3), 1.99 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 193.2, 160.9, 152.9, 143.2, 142.3, 141.1, 139.0, 129.8, 129.4, 125.9, 125.2, 124.3, 117.2, 93.5, 40.9, 39.2, 21.6, 19.1; IR (KBr) ν: 3453, 1684, 1593, 1573, 1490, 1317, 1254 cm-1; HRMS (TOF ES+) calcd for C20H18N3O4 [M+H]+ 364.1297, found 364.1293.
11-(4-甲氧基苯甲酰基)-8-硝基-3, 4-二氢-1H-嘧啶并[1, 2-b]异喹啉-6(2H)-酮(3j):黄色固体, m.p. 25~253 ℃; Rf=0.3 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (500 MHz, DMSO-d6) δ: 9.92 (s, 1H, NH), 8.74 (s, 1H, ArH), 7.87 (d, J=9.0 Hz, 1H, ArH), 7.55 (d, J=7.5 Hz, 2H, ArH), 6.95 (d, J=7.5 Hz, 2H, ArH), 6.85 (d, J=9.0 Hz, 1H, ArH), 4.08 (s, 2H, CH2), 3.45 (s, 2H, CH2), 2.05 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 192.5, 162.8, 160.9, 152.4, 143.2, 140.9, 133.8, 131.7, 125.6, 125.4, 124.4, 117.0, 114.4, 93.5, 55.9, 40.9, 39.2, 19.2; IR (KBr) ν: 3462, 1683, 1594, 1574, 1478, 1315, 1256 cm-1; HRMS (TOF ES+) calcd for C20H18N3O5 [M+H]+ 380.1246, found 380.1240.
11-(4-氟苯甲酰基)-9-硝基-3, 4-二氢-1H-嘧啶并[1, 2-b]异喹啉-6(2H)-酮(3k):黄色固体. m.p. 258~260 ℃; Rf=0.28 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (600 MHz, DMSO-d6) δ: 10.83 (s, 1H, NH), 8.19~8.21 (m, 1H, ArH), 7.71~7.72 (m, 1H, ArH), 7.56~7.58 (m, 2H, ArH), 7.49~7.50 (m, 1H, ArH), 7.25~7.28 (m, 2H, ArH), 4.07 (t, J=5.8 Hz, 2H, CH2), 3.50 (s, 2H, CH2), 2.06 (t, J=4.7 Hz, 2H, CH2); 13C NMR (150 MHz, DMSO-d6) δ: 190.8, 160.7, 153.2, 149.2, 138.8, 131.5 (d, J=9.0 Hz), 129.9, 122.2, 120.4, 116.2 (d, J=22.5 Hz), 115.4, 92.3, 40.9, 38.9, 19.2; IR (KBr) ν: 3441, 1670, 1609, 1570, 1523, 1347, 1240 cm-1; HRMS (TOF ES+) calcd for C19H13FN3O4 [M-H]+ 366.0890, found 366.0897.
11-4-氯苯甲酰基)-9-硝基-3, 4-二氢-1H-嘧啶并[1, 2-B]异喹啉-6(2H)-酮(3l):黄色固体, m.p. 255~257 ℃; Rf=0.27 [V(石油醚):V(乙酸乙酯)=1:2]; 1H NMR (600 MHz, DMSO-d6) δ: 10.88 (s, 1H, NH), 8.19~8.21 (m, 1H, ArH), 7.72~7.73 (m, 1H, ArH), 7.49~7.52 (m, 5H, ArH), 4.07 (t, J=5.9 Hz, 2H, CH2), 3.50 (d, J=1.5 Hz, 2H, CH2), 2.06~2.09 (m, 2H, CH2); 13C NMR (150 MHz, DMSO-d6) δ: 190.6, 160.7, 153.4, 149.2, 141.1, 138.4, 136.1, 130.7, 129.9, 129.4, 122.3, 120.4, 115.6, 92.4, 40.9, 38.9, 19.2; IR (KBr) ν: 3445, 1673, 1604, 1573, 1529, 1343, 1242 cm-1; HRMS (TOF ES+) calcd for C19H13ClN3O4 [M-H]+ 382.0595, found 382.0601.
辅助材料(Supporting Information) 化合物3的1H NMR, 13C NMR和HRMS谱图数据.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
Zhang, A. H.; Jiang, N.; Gu, W.; Ma, J.; Wang, Y. R.; Song, Y. C.; Tan, R. X. Chem. Eur. J. 2010, 16, 14479. http://new.med.wanfangdata.com.cn/Paper/Detail?id=PeriodicalPaper_PM21038331
-
[2]
(a) Chern, M. S.; Li, W. R. Tetrahedron Lett. 2004, 45, 8323.
(b) Gonzalez, D.; Martinot, T.; Hudlicky, T. Tetrahedron Lett. 1999, 40, 3077.
(c) Thompson, R. C.; Kallmerten, J. J. Org. Chem. 1990, 55, 6076.
(d) Shamma, M.; Foy, J. E. Tetrahedron Lett. 1975, 16, 2249.
(e) Glushkov, V. A.; Shklyaev, Y. V. Chem. Heterocycl. Compd. 2001, 37, 663.
(f) Okomoto, T.; Torii, Y.; Isogai, Y. Chem. Pharm. Bull. 1968, 16, 1860. -
[3]
Bollini, M.; González, M.; Bruno, A. M. Tetrahedron Lett. 2009, 50, 1507. http://www.sciencedirect.com/science/article/pii/S004040390900135X
-
[4]
Bollini, M.; Casal, J. J.; Alvarez, D. E.; Boiani, L.; González, M.; Cerecetto, H.; Bruno, A. M. Bioorg. Med. Chem. 2009, 17, 1437. http://www.sciencedirect.com/science/article/pii/S0968089609000480
-
[5]
Sa, E.; Jutta, H.; Peter, S.; Patrick, R.; Daniel, M. WO 2012/104305, 2012[Chem. Abstr. 2012, 157, 356574].
-
[6]
(a) Senda, S.; Ohtani, O.; Katho, E.; Nagasaka, M.; Miyake, H.; Fujiwara, K.; Tanaka, M. FR 2502619, 1982[Chem. Abstr. 1983, 98, 71955].
(b) Senda, S.; Ohtani, O.; Katho, E.; Nagasaka, M.; Miyake, H.; Fujiwara, K.; Tanaka, M. DE 3211501, 1982[Chem. Abstr. 1983, 98, 198048]. -
[7]
Sulkovski, T. S.; Wille, M. A. US 3452027, 1969[Chem. Abstr. 1969, 71, 112830].
-
[8]
Kubo, K.; Ito, N.; Souzu, I.; Isomura, Y.; Homma, H. DE 2828528, 1979 [Chem. Abstr. 1979, 90, 168468].
-
[9]
Senda, O.; Ohtani, O.; Katho, E.; Miyake, H.; Fujiwara, K. DE 3031574, 1981[Chem. Abstr. 1981, 95, 132692].
-
[10]
(a) Ashton, M. J.; Bridge, A. W.; Chambers, R. K.; Dron, D. I.; Fenton, G.; Harris, N. V. EP 326386, 1989[Chem. Abstr. 1990, 112, 158072].
(b) Natsugari, H.; Ikeda, H. JP 03112967, 1991[Chem. Abstr. 1991, 115, 158988].
(c) Tang, X.-C.; He, X.-C.; Bai, D.-L. Drugs Future 1999, 24, 647.
(d) Hunt, A. H.; Mynderse, J. S.; Samlaska, S. K.; Fukuda, D. S.; Maciak, G. M.; Kirst, H. A.; Occolowitz, J. L.; Swartzendruber, J. K.; Jones, N. D. J. Antibiot. 1988, 41, 771. -
[11]
Zheng, Z.; Alper, H. Org. Lett. 2008, 10, 829.
-
[12]
Robison, M. M.; Robison, B. L. J. Org. Chem. 1956, 21, 1337. doi: 10.1021/jo01118a001
-
[13]
(a) Georgian, V. ; Harrisson, R. J. ; Skaletzky, L. L. J. Org. Chem. 1962, 27, 4571.
(b) Yu, X. ; Zhou, F. ; Chen, J. ; Xiao, W. Acta Chim. Sinica 2017, 75, 86 (in Chinese).
(余晓叶, 周帆, 陈加荣, 肖文精, 化学学报, 2017, 75, 86. ) -
[14]
Rinner, U.; Hillebrenner, H. L.; Adams, D. R.; Hudlicky, T.; Pettit, G. R. Bioorg. Med. Chem. Lett. 2004, 14, 2911. http://www.ncbi.nlm.nih.gov/pubmed/15125958
-
[15]
(a) Huang, Z.-T.; Wang, M.-X. Heterocycles 1994, 37, 1233.
(b) Huang, Z.-T.; Wang, M.-X. Prog. Nat. Sci. 1999, 11, 971.
(c) Wang, K.-M.; Yan, S.-J.; Lin, J. Eur. J. Org. Chem. 2014, 1129. -
[16]
(a) Zhou, B. ; Liu, Z. -C. ; Qu, W. -W. ; Yang, R. ; Lin, X. -R. ; Yan, S. -J. ; Lin, J. Green Chem. 2014, 16, 4359.
(b) Yu, F. -C. ; Huang, R. ; Ni, X. -C. ; Fan, J. ; Yan, S. -J. ; Lin, J. Green Chem. 2013, 15, 453.
(c) Chen, X. -B. ; Liu, Z. -C. ; Yang, L. -F. ; Yan, S. -J. ; Lin, J. ACS Sustainable Chem. Eng. 2014, 2, 1155.
(d) Chen, X. -B. ; Liu, Z. -C. ; Lin, X. -R. ; Huang, R. ; Yan, S. -J. ; Lin, J. ACS Sustainable Chem. Eng. 2014, 2, 2391.
(e) Kong, L. ; Yang, R. ; Du, X. ; Yan, S. ; Lin, J. Chin. J. Org. Chem. 2016, 36, 2437 (in Chinese).
(孔令斌, 杨瑞霞, 杜璇璇, 严胜骄, 林军, 有机化学, 2016, 36, 2437. ) -
[17]
(a) Chen, X. -B. ; Liu, X. -M. ; Huang, R. ; Yan, S. -J. ; Lin, J. Eur. J. Org. Chem. 2013, 4607.
(b) Yu, F. -C. ; Chen, Z. -Q. ; Hao, X. -P. ; Yan, S. -J. ; Huang, R. ; Lin, J. RSC Adv. 2014, 4, 6110.
(c) Ma, Y. -L. ; Wang, K. -M. ; Lin, X. -R. ; Yan, S. -J. ; Lin, J. Tetrahedron 2014, 70, 6578.
(d) Wang, K. -M. ; Ma, Y. -L. ; Lin, X. -R. ; Yan, S. -J. ; Lin, J. RSC Adv. 2015, 5, 36472.
(e) Yang, R. ; Zhao, Y. ; Jiang, M. ; Yan, S. ; Lin, J. Chin. J. Org. Chem. 2016, 36, 2941 (in Chinese).
(杨瑞霞, 赵宇澄, 蒋美妤, 严胜骄, 林军, 有机化学, 2016, 36, 2941. ) -
[18]
(a) Chen, N. ; Meng, X. ; Zhu, F. ; Cheng, J. ; Shao, X. ; Li, Z. J. Agric. Food Chem. 2015, 63, 1360.
(b) Bao, H. ; Shao, X. ; Zhang, Y. ; Deng, Y. ; Xu, X. ; Liu, Z. ; Li, Z. J. Agric. Food Chem. 2016, 64, 5148.
(c) Luo, D. ; Cui, S. ; Hu, X. ; Lin, J. ; Yan, S. Chin. J. Org. Chem. 2017, 37, 166 (in Chinese).
(罗大云, 崔时胜, 胡兴梅, 林军, 严胜骄, 有机化学, 2017, 37, 166. ) -
[19]
(a) Li, M.; Shao, P.; Wang, S.-W.; Kong, W.; Wen, L.-R. J. Org. Chem. 2012, 77, 8956.
(b) Li, M.; Zhou, Z.-M.; Wen, L.-R.; Qiu, Z.-X. J. Org. Chem. 2011, 76, 3054. -
[20]
(a) Zhang, J. H.; Wang, M.-X.; Huang, Z.-T. J. Chem. Soc., Perkin Trans. 1 1999, 321.
(b) Yaqub, M.; Yu, C.-Y.; Jia, Y.-M.; Huang, Z.-T. Synlett 2008, 1357. -
[21]
(a) Zhang, J.-H.; Wang, M.-X.; Huang, Z.-T. J. Chem. Soc., Perkin Trans. 1 1999, 2087.
(b) Liao, J.-P.; Zhang, T.; Yu, C.-Y.; Huang, Z.-T. Synlett 2007, 761. -
[22]
(a) Huang, Z.-T.; Wang, M.-X. Synthesis 1992, 1273.
(b) Li, Z.-J.; Charles, D. Synth. Commun. 2001, 31, 527.
(c) Chen, X.-B.; Liu, X.-M.; Huang, R.; Yan, S.-J.; Lin, J. Eur. J. Org. Chem. 2013, 4607.
-
[1]
-
表 1 优化反应条件a
Table 1. Optimization of reaction conditions

Entry Solvent Catalyst T/℃ Time/h Yieldb/% 1 EtOH — r.t. 12 — 2 DCM — r.t. 12 — 3 EtOH — Reflux 12 — 4 DMF — Reflux 12 — 5 Toluene — Reflux 12 20 6 1, 4-Dioxane — Reflux 12 40 7 CH3CN — Reflux 12 — 8c 1, 4-Dioxane K2CO3 Reflux 12 30 9c 1, 4-Dioxane Et3N Reflux 12 — 10c 1, 4-Dioxane Piperidine Reflux 12 — 11c 1, 4-Dioxane Cs2CO3 Reflux 12 91 12c 1, 4-Dioxane Cs2CO3 Reflux 8 70 13c 1, 4-Dioxane Cs2CO3 Reflux 20 90 14d 1, 4-Dioxane Cs2CO3 Reflux 12 92 15e 1, 4-Dioxane Cs2CO3 Reflux 12 90 aReactions were carried out using 1a (1.1 mmol), 2c (1.0 mmol), and solvent (15 mL). bIsolated yield based on HKA 2c.c Catalyst 1.0 mmol. d Catalyst 0.05 mmol. e Catalyst 0.1 mmol. 表 2 一锅法合成化合物3a
Table 2. One-pot protocol for the synthesis of compound 3

Entry 1 n Ar 3 Yieldb/% 1 1a 1 4-Fluorophenyl 3a 98 2 1a 1 4-Chlorophenyl 3b 92 3 1a 1 Phenyl 3c 92 4 1a 1 4-Methylphenyl 3d 90 5 1a 1 4-Methoxyphenyl 3e 90 6 1a 2 4-Fluorophenyl 3f 95 7 1a 2 4-Chlorophenyl 3g 93 8 1a 2 Phenyl 3h 90 9 1a 2 4-Methylphenyl 3i 92 10 1a 2 4-Methoxyphenyl 3j 90 11 1b 2 4-Fluorophenyl 3k 43 12 1b 2 4-Chlorophenyl 3l 40 aAll reactions were run under the following conditions: 1 (1.1 mmol) and 2 (1.0 mmol) were refluxed in the solvent of 1, 4-dioxane (15 mL) for 12 h. bIsolated yield based on HKAs (2). -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 53
- 文章访问数: 4058
- HTML全文浏览量: 546



 下载:
下载:

 下载:
下载:

