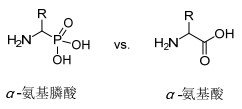
 图1
α-氨基酸和α-氨基膦酸的结构
Figure1.
Structures of amino acids and their analogue α-aminophosphonic acids
图1
α-氨基酸和α-氨基膦酸的结构
Figure1.
Structures of amino acids and their analogue α-aminophosphonic acids

Citation: Huang Xiaoli, Ren Linjing, Pu Jiazhi, He Chunyang, Yao Qiuli. An Access to the gem-Diamine Derivatives from α-Carbamatemethylphosphonate[J]. Chinese Journal of Organic Chemistry, 2017, 37(9): 2369-2376. doi: 10.6023/cjoc201702016

由α-氨基亚甲基膦酸酯构建偕二胺衍生物
-
关键词:
- α-氨基亚甲基膦酸酯
- / 偕二胺衍生物
- / 磺砜
English
An Access to the gem-Diamine Derivatives from α-Carbamatemethylphosphonate
-
Key words:
- α-carbamatemethylphosphonate
- / gem-diamine derivatives
- / sulfone
-
α-氨基膦酸是天然氨基酸的结构类似物(图 1), 这一类型化合物具有特殊的化学性质, 并且具有广泛多样的生物性质, 如抗菌性、抗HIV、抗癌活性等等[1~3], 因此, 其合成及活性研究一直是有机化学领域研究的热点[4~6].
在前期研究α-氨基亚甲基膦酸酯3a由磺砜1a与磷酸二乙酯2a反应合成的过程当中[7], 我们意外地发现将磷酸二乙酯2a的用量由0.95 equiv.降低至0.5 equiv.时, 该反应会以26%的分离产率生成偕二胺衍生物4a (Eq. 1).化合物4a独特的化学结构引起了我们的兴趣, 研究表明偕二胺衍生物由于其活泼的偕二胺结构而具有丰富的生物学活性, 如作为抗癌试剂[8, 9]、糖苷酶抑制剂、糖基转移酶抑制剂[10, 11]、HIV-1蛋白酶抑制剂[12]、鸦片受体抑制剂等[13, 14], 其合成[15~17]及活性研究是有机化学及生物化学的研究热点; 而α-氨基亚甲基膦酸酯的偕二胺衍生物可作为激酶抑制剂表现出抗癌活性[18, 19]; 但是在文献中其合成研究的报道较少, 主要是对环状的偕二胺[20~22]或二亚胺化合物进行磷酰化[23]或者醛的缩合反应[24, 25].基于上述背景, 我们决定进一步考察α-氨基亚甲基膦酸酯偕二胺衍生物的合成方法学.
1 结果与讨论
我们首先以磺砜1a与磷酸二乙酯2a (0.2 equiv.)的反应来进行反应条件的筛选(表 1).以LiOH为碱, EtOAc为溶剂, 在室温下反应9 h, 与Eq. 1结果一致的是, 进一步降低2a的用量能以更高的分离产率(41%)合成偕二胺衍生物4a (表 1, Entry 1);但是将LiOH换为碱性更弱的K2CO3或碱性更强的NaOH或KOH均无产物或只获得<5%的产率(表 1, Entries 2~4).将2a的用量降低至0.14 equiv.或升高至0.25或0.33 equiv.对产率的影响极少(表 1, Entries 5~7).将溶剂换为极性更大的DMF或中等极性的CH3CN、丙酮或THF等, 产率均降低(表 1, Entries 8~11);极性更小的DCM、DCE、Et2O或甲苯作为溶剂产率也降低(表 1, Entries 12~15);而使用极性很小的正己烷或石油醚时产率分别提高至47%和50%(表 1, Entries 16, 17).进一步以石油醚为溶剂, 将反应温度升高至50 ℃或降低至0 ℃均使产率降低(表 1, Entries 18, 19).

Entry Base Solvent Time/h Yieldb/% 1 LiOH EtOAc 9 41 2 K2CO3 EtOAc 9 <5 3 NaOH EtOAc 9 <5 4 KOH EtOAc 9 0 5b LiOH EtOAc 9 41 6c LiOH EtOAc 9 39 7d LiOH EtOAc 9 39 8 LiOH DMF 9 15 9 LiOH CH3CN 9 13 10 LiOH CH3COCH3 9 25 11 LiOH THF 9 32 12 LiOH DCM 9 14 13 LiOH DCE 9 27 14 LiOH Et2O 9 30 15 LiOH PhCH3 9 36 16 LiOH n-Hexane 9 47 17 LiOH Petroleum ether 9 50 18e LiOH Petroleum ether 9 9 19f LiOH Petroleum ether 9 20 20g LiOH Petroleum ether 5 83 a Reaction conditions: base (2 mmol), 1a (1 mmol), 2a (0.2 mmol), solvent (3 mL), at room temperature under Ar; isolated yield after column chromatography. b 2a (0.14 mmol); c 2a (0.25 mmol); d 2a (0.33 mmol); eat 50 ℃; f at 0 ℃; g 3a (0.25 mmol) was used instead of 2a. 从实验结果我们推测, 偕二胺衍生物4a可能是由中间产物3a与1a进一步反应而得到的, 因此, 我们用α-氨基亚甲基膦酸酯3a (0.25 equiv.)代替磷酸二乙酯2a时反应5 h后薄层色谱(TLC)显示底物3a转化完全, 此时分离产率能提高至83% (表 1, Entry 20), 理论上4a仍能继续与一分子或多分子1a反应生成三胺基或多胺基取代的膦酸酯, 不过在此条件下未分离得到这些副产物.
在获得以上优化结果的基础上, 我们进一步考察底物的适用性, 合成其他α-氨基亚甲基膦酸酯的偕二胺衍生物(表 2).以Boc或Cbz保护的氨基亚甲基膦酸酯3a~3e均可顺利地与苯磺砜1a或1b反应, 可获得良好的产率(49%~87%).



aReaction conditions: LiOH (2 mmol), 1 (1 mmol), 3 (0.25 mmol), petroleum ether (3 mL), at room temperature under Ar; isolated yield after column chromatography. 考虑到使用对甲苯磺酰基(Ts)作为离去基团可能会由于苯环对位有给电性的甲基取代, 使得底物的活性有所降低, 从而提高这个底物的稳定性.因此Ts取代的底物1c~1f被用到底物拓展当中(表 3).令人高兴的是, 无论是亚甲基磺砜(1c、1d, 产率41%~86%)还是具有苯基或丁基或乙基取代的磺砜(1e~1f, 产率55%~94%), 该类底物同样能顺利地转化为偕二胺衍生物4, 产率普遍优秀, 从实验结果来看, 有苯基或丁基或乙基取代的磺砜反应的产率普遍比亚甲基磺砜高, 这可能与反应中间体的稳定性有关[7].该反应可能涉及1转化为亚胺中间体, 该中间体继续与3反应, 有苯基等取代的亚胺稳定性比无取代的亚胺要高, 因而前者产率高.






a Reaction conditions: LiOH (2 mmol), 1 (1 mmol), 3 (0.25 mmol), petroleum ether (3 mL), at room temperature under Ar; isolated yield after column chromatography. 该反应的机理可能是在碱性条件下底物1的磺砜基离去形成亚胺中间体, 化合物3的酰胺基具有较弱的亲核性, 其与亚胺中间体反应进而得到最终产物偕二胺4.
2 结论
综上所述, 本研究为α-氨基亚甲基膦酸酯的偕二胺衍生物提供了一种条件温和、操作简便、高产率的合成方法.本方法只需要以温和的氢氧化锂作为碱, 使用无需特殊处理的石油醚作为溶剂, 具有良好的底物适用性, 为进一步对该类化合物化学及生物学活性的考察奠定了基础.
3 实验部分
3.1 仪器与试剂
核磁共振仪为Agilent-NMR-v NMRs 400型, CDCl3为溶剂, 以TMS为内标; 高分辨质谱仪为Thermo Scientific LTQ Orbitrap XL型(ESI-HRMS).薄层层析板和硅胶购自青岛海洋化工厂.氢氧化锂购自阿达玛斯试剂有限公司.所用试剂为分析纯级别, 获得后直接使用, 未纯化.
3.2 实验操作
取25 mL干燥的圆底烧瓶, 在氩气保护下加入氢氧化锂(48 mg, 2 mmol)、化合物1 (271 mg, 1 mmol), 再次抽换氩气后加入化合物3 (0.25 mmol)及石油醚(3 mL), 室温下反应, TLC显示反应完全之后过滤、浓缩, 通过快速柱层析纯化[V(石油醚): V(乙酸乙酯)=3: 1至1: 1) 得到产物4.由于产物4的酰胺键具有部分烯醇式的顺反异构体, 因此该类化合物部分氢、碳以及磷显示不等价的信号; 对于这些异构体同一个H或C或P的两个信号在文中用and来表示.
偕二胺4a: 82或80 mg, 产率83%或80%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 5.55 (br s, 1H), 4.56 (s, 2H), 4.05~4.00 (m, 4H), 3.70 (d, 2JP—H=8 Hz, 2H, CH2-P), 1.35 (s, 9H), 1.31 (s, 9H), 1.21~1.20 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ: 156.0, 154.6 (NHCO), 155.2, 154.2 (NCO), 80.5, 79.0, 62.0 (d, 2JP—C=7 Hz, P-O-CH2) and 61.8 (d, 2JP—C=6 Hz, P-O-CH2), 54.0 and 53.3 (N-CH2-NH), 42.2 (d, 1JP—C=157 Hz, CH2-P) and 41.3 (d, 1JP—C=156 Hz, CH2-P), 28.0, 27.9, 16.1~16.0 (m); 31P NMR (CDCl3, 162 MHz) δ: 23.2 and 22.9; IR (film) ν: 3348, 2927, 1703, 1248, 1154, 1053, 1026, 774 cm-1; HRMS (ESI) calcd for C16H34N2O7P [M+H]+ 397.2098, found 397.2097.
偕二胺4b: 70或82 mg, 产率60%或71%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 5.54 (br s, 1H), 4.63 (s, 2H), 4.04~4.02 (m, 4H), 3.78 (d, 2J(P—H)=8 Hz, 2H, CH2-P), 1.61 (br s, 4H), 1.43 (s, 9H), 1.39 (s, 9H), 1.35 (m, 4H), 0.90~0.88 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ: 156.0 and 155.0 (NHCO), 155.3 and 154.5 (NCO), 81.0, 79.6, 66.0 (d, 2JP—C=6 Hz, P-O-CH2) and 65.8 (d, 2JP—C=7 Hz, P-O-CH2), 54.5 and 53.8 (N-CH2-NH), 42.6 (d, 1JP—C=157 Hz, CH2-P) and 41.8 (d, 1JP—C=157 Hz, CH2-P), 32.53 (d, 3JP—C=6 Hz, P-O-CH2-CH2) and 32.46 (d, 3JP—C=6 Hz, P-O-CH2-CH2), 28.3, 18.6, 13.6; 31P NMR (CDCl3, 162 MHz) δ: 23.3 and 23.0; IR (film) ν: 3353, 2962, 1703, 1248, 1154, 1062, 1025 cm-1. HRMS (ESI) calcd for C20H42N2O7P [M+H]+ 453.2724, found 453.2723.
偕二胺4c: 82或80 mg, 产率77%或74%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.33 (br s, 5H), 5.62 and 5.49 (s, 1H, NH), 5.15 and 5.13 (s, 2H, Ph-CH2), 4.73~4.71 (m, 2H), 4.11~4.02 (m, 4H), 3.88 (d, 2J(P—H)=8 Hz, 2H, CH2-P), 1.41 (s, 9H), 1.26~1.22 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ: 156.0 and 155.6 (NHCO), 155.8 and 155.4 (NCO), 136.1 and 135.8 (ipso-C), 128.53 and 128.49 (meta-CH), 128.2 (para-CH), 128.1 and 128.0 (ortho-CH), 79.9, 67.8, 62.4 (d, 2JP—C=7 Hz, P-O-CH2) and 62.3 (d, 2JP—C=7 Hz, P-O-CH2), 54.4 and 54.1 (N-CH2-NH), 43.3 and 41.7 (CH2-P), 28.3, 16.3 (d, 3JP—C=5 Hz, P-O-CH2-CH3); 31P NMR (CDCl3, 162 MHz) δ: 23.8 and 23.3; IR (film) ν: 3358, 3063, 2927, 2363, 1708, 1245, 1158, 1053, 1026 cm-1; HRMS (ESI) calcd for C19H32N2O7P [M+H]+ 431.1942, found 431.1940.
偕二胺4d: 87 mg, 产率86%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.31 (s, 5H), 5.71 and 5.49 (s, 1H, NH), 5.12 and 5.11 (s, 2H, Ph-CH2), 4.68 (s, 2H), 3.88 (d, 2J(P—H)=8 Hz, 2H, CH2-P), 3.71 (d, 3J(P—H)=12 Hz, 3H, OCH3), 3.61 (d, 3J(P—H)=12 Hz, 3H, OCH3), 1.38 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ: 156.2 and 155.7 (NHCO), 155.7 and 155.4 (NCO), 136.0 and 135.8 (ipso-C), 128.52 and 128.49 (meta-CH), 128.3 (para-CH), 128.17 and 127.9 (ortho-CH), 80.00 and 79.95 (C(CH3)3), 67.9, 54.3 and 54.0 (N-CH2-NH), 52.82 (d, 2JP—C=12 Hz, P-O-CH3) and 52.76 (d, 2JP—C=14 Hz, P-O-CH3), 42.0 (d, 1JP—C=156 Hz, CH2-P), 28.2; 31P NMR (CDCl3, 162 MHz) δ: 25.6 and 25.0; IR (film) ν: 3348, 3063, 2925, 1705, 1247, 1154, 1114, 1027 cm-1; HRMS (ESI) calcd for C17H28N2-O7P [M+H]+ 403.1629, found 403.1628.
偕二胺4e: 68 mg或100 mg, 产率56%或82%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.31 (s, 5H), 5.69 and 5.54 (s, 1H, NH), 5.13 and 5.10 (s, 2H, Ph-CH2), 4.70 (s, 2H), 4.01~3.95 (m, 4H), 3.87 (d, 2J(P—H)=8 Hz, 2H, CH2-P), 1.57~1.53 (m, 4H), 1.38 (s, 9H), 1.33~1.21 (m, 4H), 0.87~0.85 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ: 156.0 and 155.5 (NHCO), 155.8 and 155.4 (NCO), 136.1 and 135.8 (ipso-C), 128.5, 128.2, 128.0 and 127.9 (ortho-CH), 79.8, 67.8, 66.1 (d, 2JP—C=7 Hz, P-O-CH2) and 65.9 (d, 2JP—C=7 Hz, P-O-CH2), 54.4 and 54.1 (N-CH2-NH), 42.4 (d, 1JP—C=158 Hz, CH2-P), 32.4 (d, 3JP—C=6 Hz, P-O-CH2-CH2), 28.2, 18.6, 13.5; 31P NMR (CDCl3, 162 MHz) δ: 22.8 and 22.4; IR (film) ν: 3391, 3071, 2925, 1707, 1252, 1143, 1075, 1040 cm-1; HRMS (ESI) calcd for C23H40N2O7P [M+H]+ 487.2568, found 487.2567.
偕二胺4f: 52或56 mg, 产率49%或52%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.31 (br s, 5H), 5.96 (s, 1H), 5.13 and 5.07 (s, 2H, Ph-CH2), 4.73~4.70 (m, 2H, NH-CH2), 4.12~4.09 (m, 4H), 3.81 (d, 2J(P—H)=8 Hz, 2H, CH2-P), 1.42 (s, 9H), 1.28 (s, 3H), 1.22 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ: 156.8 and 154.9 (NHCO), 156.1 and 154.4 (NCO), 136.2, 128.5, 128.1, 128.0, 81.2, 66.8, 62.4 (d, 2JP—C=6 Hz, P-O-CH2) and 62.2 (d, 2JP—C=6 Hz, P-O-CH2), 54.9 and 54.3 (N-CH2-NH), 42.9 (d, 1JP—C=158 Hz, CH2-P) and 42.0 (d, 1JP—C=157 Hz, CH2-P), 28.2, 16.4 (d, 3JP—C=6 Hz, P-O-CH2-CH3) and 16.3 (d, 3JP—C=5 Hz, P-O-CH2-CH3); 31P NMR (CDCl3, 162 MHz) δ: 23.4 and 23.1; IR (film) ν: 3261, 3034, 2979, 1703, 1243, 1157, 1054, 1028 cm-1; HRMS (ESI) calcd for C19H32N2O7P[M+H]+ 431.1942, found 431.1942.
偕二胺4g: 78或61 mg, 产率68%或53%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.28 (s, 10H), 6.33 and 6.10 (s, 1H, NH), 5.12~5.05 (m, 4H), 4.80~4.76 (m, 2H, NH-CH2), 4.08~3.97 (m, 4H), 3.89 (d, 2J(P—H)=12 Hz, 2H, CH2-P), 1.24~1.17 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ: 157.0 and 155.7 (NHCO), 156.3 and 155.3 (NCO), 136.2, 136.1 and 135.8 (ipso-C), 128.5, 128.2~128.0 (m), 67.9, 66.9, 62.5 (d, 2JP—C=6 Hz, P-O-CH2) and 62.3 (d, 2JP—C=6 Hz, P-O-CH2), 54.8 and 54.5 (N-CH2-NH), 42.6 (d, 1JP—C=157 Hz, CH2-P) and 42.5 (d, 1JP—C=156 Hz, CH2-P), 16.3 (d, 3JP—C=3 Hz, P-O-CH2-CH3); 31P NMR (CDCl3, 162 MHz) δ: 22.8 and 22.3; IR (film) ν: 3358, 3065, 2925, 1712, 1243, 1180, 1054, 1025 cm-1; HRMS (ESI) calcd for C22H30N2O7P [M+H]+ 465.1785, found 465.1783.
偕二胺4h: 113或81 mg, 产率87%或62%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.33~7.32 (m, 10H), 6.07 and 5.92 (s, 1H, NH), 5.15~5.07 (m, 4H), 4.82~4.78 (m, 2H), 4.04~3.96 (m, 4H), 3.91 (d, 2J(P—H)=8 Hz, 2H, CH2-P), 1.59~1.54 (m, 4H), 1.36~1.30 (m, 4H), 0.91~0.86 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ: 156.8 and 155.7 (NHCO), 156.2 and 155.3 (NCO), 136.1, 136.0 and 135.8 (ipso-C), 128.5, 128.3~128.0 (m), 67.9, 67.0, 66.1 (d, 2JP—C=7 Hz, P-O-CH2) and 66.0 (d, 2JP—C=6 Hz, P-O-CH2), 54.9 and 54.6 (N-CH2-NH), 42.6 (d, 1JP—C=157 Hz, CH2-P) and 42.6 (d, 1JP—C=157 Hz, CH2-P), 32.5 (d, 3JP—C=6 Hz, P-O-CH2-CH2), 18.6, 13.6; 31P NMR (CDCl3, 162 MHz) δ: 22.7 and 22.3; IR (film) ν: 3374, 3034, 2925, 1710, 1242, 1180, 1075, 1027 cm-1; HRMS (ESI) calcd for C26H38N2O7P [M+H]+ 521.2411, found 521.2410.
偕二胺4i: 57或44 mg, 产率52%或41%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.33~7.32 (m, 10H), 6.06 and 5.84 (s, 1H, NH), 5.15~5.08 (m, 4H), 4.80~4.76 (m, 2H), 3.92 (d, 2J(P—H)=8 Hz, 2H, CH2-P), 3.73 (d, 3J(P—H)=8 Hz, 3H, OCH3), 3.64 (d, 3J(P—H)=12 Hz, 3H, OCH3); 13C NMR (CDCl3, 100 MHz) δ: 156.9 and 155.7 (NHCO), 156.4 and 155.4 (NCO), 136.1, 135.9 and 135.8 (ipso-C), 128.5, 128.3, 128.2~128.0 (m), 68.1 and 68.0 (Ph-CH2O-CON), 67.0, 54.8 and 54.5 (N-CH2-NH), 52.9 (d, 2JP—C=6 Hz, P-O-CH3) and 52.8 (d, 2JP—C=6 Hz, P-O-CH3), 41.8 (d, 1JP—C=157 Hz, CH2-P); 31P NMR (CDCl3, 162 MHz) δ: 25.5 and 24.9; IR (film) ν: 3347, 3063, 2924, 1704, 1239, 1186, 1142, 1027 cm-1; HRMS (ESI) calcd for C20H26N2O7P[M+H]+ 437.1472, found 437.1470.
偕二胺4j: 54 mg, 产率54%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.33~7.31 (m, 5H), 5.90 and 5.83 (s, 1H, NH), 5.08 (s, 2H), 4.71 (s, 2H), 3.84 (d, 2J(P—H)=12 Hz, 2H, CH2-P), 3.75 (d, 3J(P—H)=8 Hz, 6H, OCH3), 1.44 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ: 156.9 and 154.9 (NHCO), 156.3 and 154.5 (NCO), 136.1, 128.5, 128.2, 128.1, 81.5, 67.0, 54.9 and 54.3 (N-CH2-NH), 53.0~52.8 (m, P-O-CH3), 42.2 (d, 1JP—C=158 Hz, CH2-P) and 41.5 (d, 1JP—C=154 Hz, CH2-P), 28.2; 31P NMR (CDCl3, 162 MHz) δ: 26.0 and 25.5; IR (film) ν: 3335, 3058, 2926, 1703, 1243, 1155, 1030 cm-1; HRMS (ESI) calcd for C17H28N2O7P [M+H]+ 403.1629, found 403.1629.
偕二胺4k: 61 mg, 产率55%, 无色油状物.1H NMR (CDCl3, 400 MHz) δ: 7.35~7.26 (m, 5H), 6.70~6.65 (s, 1H, NH), 6.65 (s, 1H), 4.03~4.01 (m, 1H), 3.76 (d, 3J(P—H)=12 Hz, 3H, OCH3), 3.69 (d, 3J(P—H)=8 Hz, 3H, OCH3), 3.23 (br s, 1H), 1.46 (s, 9H), 1.39 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ: 155.0, 154.6, 138.3, 128.5, 127.8, 126.2, 81.7, 79.7, 66.4, 53.0 (d, 2JP—C=5 Hz, P-O-CH3) and 52.7 (d, 2JP—C=5 Hz, P-O-CH3), 39.6 (d, 1JP—C=168 Hz, CH2-P), 28.4, 28.1; 31P NMR (CDCl3, 162 MHz) δ: 26.4 and 26.0; IR (film) ν: 3348, 3061, 2962, 1710, 1246, 1160, 1064, 1024 cm-1; HRMS (ESI) calcd for C20H34N2-O7P [M+H]+ 445.2098, found 445.2098.
偕二胺4l: 84 mg, 产率70%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.33~7.26 (m, 10H), 6.88~6.77 (m, 2H), 5.18 (s, 2H), 4.05 (br s, 1H), 3.64 (d, 3J(P—H)=12 Hz, 6H, OCH3), 3.25 (br s, 1H), 1.46 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ: 155.6, 155.0, 137.6, 135.9, 128.7, 128.4, 128.1, 127.8, 126.3, 79.9, 68.1, 67.4 and 66.3 (N-CH-NH), 53.2~52.8 (m, P-O-CH3), 40.0 (d, 1JP—C=164 Hz, CH2-P), 28.4; 31P NMR (CDCl3, 162 MHz) δ: 25.8 and 25.0; IR (film) ν: 3329, 3063, 2925, 2359, 1705, 1247, 1143, 1091, 1029 cm-1; HRMS (ESI) calcd for C23H32N2-O7P[M+H]+ 479.1942, found 479.1941.
偕二胺4m: 84 mg, 产率73%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 5.92 (br s, 1H, NH), 5.39~5.16 (m, 1H, NH-CH), 4.17~4.08 (m, 4H), 3.97 (br s, 1H), 3.54 (br s, 1H), 1.83~1.81 (m, 2H), 1.47 (s, 9H), 1.42 (s, 9H), 1.33~1.29 (m, 10H), 0.88 (t, 3JH—H=12 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 154.9, 154.6, 80.9, 79.2, 64.4 (m, N-CH-NH), 62.4~62.1 (m, P-O-CH2), 38.6 (d, 1JP—C=160 Hz, CH2-P), 33.6, 28.29, 28.26, 28.0, 22.2, 16.40~16.38 (m, P-O-CH2-CH3), 13.9; 31P NMR (CDCl3, 162 MHz) δ: 23.9 and 23.2; IR (film) ν: 3372, 2925, 1702, 1247, 1180, 1068, 1029 cm-1; HRMS (ESI) calcd for C20H42N2O7P [M+H]+ 453.2724, found 453.2724.
偕二胺4n: 100 mg, 产率79%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 5.95 (br s, 1H, NH), 5.40~5.14 (m, 1H, NH-CH), 4.06~3.99 (m, 5H P-O-CH2and P-CH2), 3.55 (br s, 1H, P-CH2), 1.89~1.77 (m, 2H), 1.67~1.59 (m, 4H), 1.46 (s, 9H), 1.41 (s, 9H), 1.39~1.33 (m, 4H), 1.30~1.24 (m, 4H), 0.93~0.86 (m, 9H); 13C NMR (CDCl3, 100 MHz) δ: 154.9, 154.6, 80.9, 79.2, 66.1~65.9 (m, P-O-CH2), 64.4~64.2 (m, N-CH-NH), 39.3 (d, 1JP—C=208 Hz, CH2-P), 33.7, 32.6~32.5 (m, P-O-CH2-CH2), 28.32, 28.29, 28.1, 22.3, 18.7, 14.0, 13.6; 31P NMR (CDCl3, 162 MHz) δ: 23.9 and 23.3; IR (film) ν: 3305, 2961, 1699, 1244, 1178, 1066, 1024 cm-1; HRMS (ESI) calcd for C24H50N2O7P [M+H]+ 509.3350, found 509.3350.
偕二胺4o: 70 mg, 产率66%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 5.77 (br s, 1H), 5.31~5.17 (m, 1H, NH-CH), 3.95~3.93 (m, 1H), 3.76~3.72 (m, 6H), 3.58~3.56 (m, 1H), 2.04~1.75 (m, 2H), 1.46 (s, 9H), 1.41 (s, 9H), 1.30~1.22 (m, 4H), 0.87 (t, 3JH—H=6 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 155.0, 154.4, 81.1, 79.4, 64.9~64.7 (m, NH-CH), 53.0~52.7 (m, P-O-CH3), 38.7 (d, 1JP—C=154 Hz, CH2-P), 33.6, 28.30, 28.28, 28.0, 22.2, 14.0; 31P NMR (CDCl3, 162 MHz) δ: 26.3; IR (film) ν: 3308, 2927, 1721, 1245, 1179, 1038, 867 cm-1; HRMS (ESI) calcd for C18H38N2O7P+ [M+H] 425.2411, found 425.2410.
偕二胺4p: 105 mg, 产率86%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.35~7.26 (m, 5H), 5.82 (br s, 1H), 5.47~5.15 (m, 3H), 4.06~4.01 (m, 5H), 3.62 (br s, 1H), 2.08~1.78 (m, 2H), 1.40 (s, 9H), 1.25~1.23 (m, 10H), 0.85 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ: 155.7, 154.8, 136.2, 128.4, 128.1, 128.0, 79.6, 67.6, 64.7, 62.3, 42.9 (d, 1JP—C=165 Hz, CH2-P) and 39.8 (d, 1JP—C=146 Hz, CH2-P), 33.6 and 33.2 (CH2-CH-NH), 28.3, 28.1, 22.2, 16.3 (d, 3JP—C=6 Hz, P-O-CH2-CH3), 14.0; 31P NMR (CDCl3, 162 MHz) δ: 23.3 and 22.4; IR (film) ν: 3331, 3065, 2927, 1708, 1217, 1166, 1028 cm-1; HRMS (ESI) calcd for C23H40N2O7P [M+H]+ 487.2568, found 487.2563.
偕二胺4q: 123 mg, 产率91%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.35~7.26 (m, 5H), 5.87 (br s, 1H), 5.46~5.15 (m, 3H), 4.00~3.96 (m, 5H), 3.64 (s, 1H), 1.89~1.80 (m, 2H), 1.59~1.53 (m, 4H), 1.41 (s, 9H), 1.35~1.24 (m, 8H), 0.91~0.87 (m, 9H); 13C NMR (CDCl3, 100 MHz) δ: 155.5, 154.9, 136.2, 128.4, 128.05, 127.95, 79.5, 67.6, 66.1~65.9 (m, P-O-CH2), 64.6, 42.8 (d, 1JP—C=162 Hz, CH2-P) and 39.7 (d, 1JP—C=142 Hz, CH2-P), 33.7 and 33.2 (CH2-CH-NH), 32.5~32.4 (m, P-O-CH2-CH2), 28.3, 28.1, 22.2, 18.6, 14.0, 13.6; 31P NMR (CDCl3, 162 MHz) δ: 23.3 and 22.5; IR (film) ν: 3303, 3065, 2960, 1713, 1242, 1174, 1066, 1027 cm-1; HRMS (ESI) calcd for C27H48N2O7P[M+H]+ 543.3194, found 543.3195.
偕二胺4r: 107 mg, 产率93%, 无色油状物.1H NMR (CDCl3, 400 MHz) δ: 7.37~7.26 (m, 5H), 5.70 (br s, 1H), 5.16~5.14 (m, 3H), 3.98 (br s, 1H), 3.68~3.66 (m, 7H), 2.02~1.77 (m, 2H), 1.41 (s, 9H), 1.28 (br s, 4H), 0.85 (br s, 3H); 13C NMR (CDCl3, 100 MHz) δ: 155.5, 155.0, 136.1, 128.5, 128.1, 128.0, 79.7, 67.7, 65.0, 52.8 (d, 2JP—C=6 Hz, P-O-CH3), 42.3 (d, 1JP—C=162 Hz, CH2-P) and 39.6 (d, 1JP—C=157 Hz, CH2-P), 33.6 and 33.2 (CH2-CH-NH), 28.3, 28.1, 22.2, 13.9; 31P NMR (CDCl3, 162 MHz) δ: 25.8 and 24.9; IR (film) ν: 3454, 3066, 2925, 1712, 1245, 1180, 1142, 1039 cm-1; HRMS (ESI) calcd for C21H36N2O7P [M+H]+ 459.2255, found 459.2254.
偕二胺4s: 81 mg, 产率77%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 5.88 (s, 1H), 5.27~5.09 (m, 1H, NH), 4.16~4.06 (m, 4H), 3.99 (br s, 1H), 3.53 (br s, 1H), 1.81 (br s, 2H), 1.46 (s, 9H), 1.41 (s, 9H), 1.31~1.28 (m, 6H), 0.91~0.88 (m, 3H); 13C NMR (CDCl3, 100 MHz) δ: 155.0, 154.5, 80.9, 79.2, 67.8~65.8 (m, NH-CH), 62.4~62.1 (m, P-O-CH2), 39.0 (d, 1JP—C=154 Hz, CH2-P), 28.29, 28.26, 27.1, 16.4~16.3 (m, P-O-CH2-CH3), 10.4; 31P NMR (CDCl3, 162 MHz) δ: 25.7 and 24.8; IR (film) ν: 3308, 2927, 1722, 1242, 1144, 1054, 1029 cm-1; HRMS (ESI) calcd for C18H38N2O7P [M+H]+ 425.2411, found 425.2412.
偕二胺4t: 92 mg, 产率87%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 5.92 (br s, 1H), 5.28~5.03 (m, 1H, NH), 4.03~4.01 (m, 5H), 3.52 (br s, 1H), 1.79 (br s, 2H), 1.60 (br s, 4H), 1.44 (s, 9H), 1.39 (s, 9H), 1.35~1.33 (m, 4H), 0.90~0.88 (m, 9H); 13C NMR (CDCl3, 100 MHz) δ: 155.0, 154.6, 80.9, 79.2, 67.8 (br s, NH-CH), 66.1~65.8 (m, P-O-CH2), 38.7 (d, 1JP—C=163 Hz, CH2-P), 32.5~32.4 (m, P-O-CH2-CH2), 28.3, 28.2, 27.1, 18.6, 13.5, 10.3; 31P NMR (CDCl3, 162 MHz) δ: 23.8 and 23.2; IR (film) ν: 3307, 2963, 1721, 1242, 1146, 1069, 1026 cm-1; HRMS (ESI) calcd for C22H46N2O7P [M+H]+ 481.3037, found 481.3037.
偕二胺4u: 108 mg, 产率94%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.35~7.26 (m, 5H), 5.81 (br s, 1H), 5.37~5.02 (m, 3H), 4.06~4.04 (m, 5H), 3.62 (br s, 1H), 1.83 (br s, 2H), 1.41 (s, 9H), 1.23 (br s, 6H), 0.9 (br s, 3H); 13C NMR (CDCl3, 100 MHz) δ: 155.4, 155.0, 136.2, 128.4, 128.1, 127.9, 79.6, 67.6, 68.7 and 66.2 (N-CH-NH), 62.3 (d, 2JP—C=5 Hz, P-O-CH2), 42.9 (d, 1JP—C=158 Hz, CH2-P) and 40.0 (d, 1JP—C=160 Hz, CH2-P), 28.3, 27.2 and 26.6 (CH2-CH-NH), 16.3 (d, 3JP—C=6 Hz, P-O-CH2-CH3), 10.4; 31P NMR (CDCl3, 162 MHz) δ: 23.2 and 22.4; IR (film) ν: 3342, 3063, 2927, 1705, 1240, 1143, 1028, 965 cm-1; HRMS (ESI) calcd for C21H36N2O7P [M+H]+ 459.2255, found 459.2255.
偕二胺4v: 99 mg, 产率92%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.31~7.24 (m, 5H), 5.73 (s, 1H), 5.29~4.97 (m, 3H), 3.97~3.95 (m, 1H), 3.64~3.62 (m, 7H), 1.76 (br s, 2H), 1.37 (s, 9H), 0.85 (br s, 3H); 13C NMR (CDCl3, 100 MHz) δ: 155.6, 155.0, 136.1, 128.5, 128.1, 128.0, 79.7, 67.7, 68.8 and 66.4 (N-CH-NH), 52.8 (d, 2JP—C=4 Hz, P-O-CH3), 42.2 (d, 1JP—C=154 Hz, CH2-P) and 39.5 (d, 1JP—C=153 Hz, CH2-P), 28.3, 27.2 and 26.5 (CH2-CH-NH), 10.4; 31P NMR (CDCl3, 162 MHz) δ: 25.7 and 24.8; IR (film) ν: 3521, 3079, 2925, 1723, 1221, 1142, 1075 cm-1; HRMS (ESI) calcd for C19H32N2O7P [M+H]+ 431.1942, found 431.1942.
偕二胺4w: 116 mg, 产率90%, 无色油状物. 1H NMR (CDCl3, 400 MHz) δ: 7.35~7.26 (m, 5H), 5.86 (br s, 1H), 5.40~5.04 (m, 3H), 4.00~3.98 (m, 5H), 3.63 (br s, 1H), 1.87 (br s, 2H), 1.58~1.56 (m, 4H), 1.42 (s, 9H), 1.34~1.25 (m, 4H), 0.90~0.88 (m, 9H); 13C NMR (CDCl3, 100 MHz) δ: 155.0, 154.4, 136.2, 128. 5, 128.1, 127.9, 79.6, 68.7, 67.7~67.5 (m, NH-CH-N), 66.2~66.0 (m, P-O-CH2), 42.7 (d, 1JP—C=164 Hz, CH2-P) and 39.8 (d, JP—C=167 Hz, CH2-P), 32.5 (d, 3JP—C=2 Hz, P-O-CH2-CH2) and 32.4 (d, 3JP—C=2 Hz, P-O-CH2-CH2), 28.3, 27.1 and 26.5 (CH2-CH-NH), 18.6 (d, 4JP—C=2 Hz, P-O-CH2-CH2-CH2), 13.6, 10.4; 31P NMR (CDCl3, 162 MHz) δ: 23.3 and 22.5; IR (film) ν: 3451, 3064, 2926, 1710, 1240, 1179, 1075, 1025 cm-1; HRMS (ESI) calcd for C25H44N2O7P [M+H]+ 515.2881, found 515.2882.
-
-
[1]
Kukuhar, V. P.; Hudson, H. R. Aminophosphorus Acids and Aminophosphinic Acids, Wiley, Chichester (UK), 2000. http://www.academia.edu/10574973/ChemInform_Abstract_Recent_Advances_in_the_Synthesis_of_Sulfonic_Acids
-
[2]
Orsini, F.; Sello, G.; Sisti, M. Curr. Med. Chem. 2010, 17, 264. doi: 10.2174/092986710790149729
-
[3]
Galezowska, J.; Gumienna-Kontecka, E. Coord. Chem. Rev. 2012, 256, 105. doi: 10.1016/j.ccr.2011.07.002
-
[4]
Wang, L.; Shen, Q.; Lu, L. Chin. J. Chem. 2013, 31, 892. doi: 10.1002/cjoc.v31.7
-
[5]
Gu, L.; Wang, R.; Huang, X.; Jin, C. Chin. J. Chem. 2012, 30, 2483. doi: 10.1002/cjoc.201200831
-
[6]
Cai, Z.; Fan, Y.; Du, G.; He, L. Chin. J. Chem. 2012, 30, 1658. doi: 10.1002/cjoc.v30.7
-
[7]
黄晓丽, 任林静, Sajjad Ali, 蒲家志, 姚秋丽, 有机化学, 2017, 37, 2073. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract346029.shtmlHuang, X. L.; Ren, L. J.; Sajjad Ali; Pu, J. Z.; Yao, Q. L. Chin. J. Org. Chem. 2017, 37, 2073(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract346029.shtml
-
[8]
Carriero, M. V.; De R., M.; Pavone, V. US 8354374, 2013[Chem. Abstr. 2013, 166, 368674].
-
[9]
Sanchez-Fernandez, E. M.; Goncalves-Pereira, R.; Risquez-Cuadro, R.; Plata, G. B.; Padron, J. M.; Garcia F. J. M.; Ortiz M. C. Carbohydr. Res. 2016, 429, 113. doi: 10.1016/j.carres.2016.01.006
-
[10]
Nishimura, Y. J. Antibiot. 2009, 62, 407. doi: 10.1038/ja.2009.53
-
[11]
Nishimura, Y.; Shitara, E.; Adachi, H.; Toyoshima, M.; Nakajima, M.; Okami, Y.; Takeuchi, T. J. Org. Chem. 2000, 65, 2. doi: 10.1021/jo982448c
-
[12]
Marastoni, M.; Bortolotti, F.; Salvadori, S.; Tomatis, R. Arzneim. Forsch. 1998, 48, 709.
-
[13]
Kondo, M.; Kitajima, H.; Yasunaga, T.; Kodama, H.; Costa, T.; Shimohigashi, Y. Bull. Chem. Soc. Jpn. 1995, 68, 3161. doi: 10.1246/bcsj.68.3161
-
[14]
Chorev, M.; Goodman, M. Acc. Chem. Res. 1993, 26, 266. doi: 10.1021/ar00029a007
-
[15]
杨锦明, 褚雪强, 纪顺俊, 有机化学, 2014, 34, 2462. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344666.shtmlYang, J.; Chu, X.; Ji, S. Chin. J. Org. Chem. 2014, 34, 2462(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344666.shtml
-
[16]
Zhang, S.; Cha, L.; Li, L.; Hu, Y.; Li, Y.; Zha, Z.; Wang, Z. J. Org. Chem. 2016, 81, 3177. doi: 10.1021/acs.joc.6b00087
-
[17]
Sanchez-F. E. M.; Alvarez, E.; Ortiz M. C.; Garcia F. J. M. J. Org. Chem. 2014, 79, 117228.
-
[18]
Cannizzaro, C.; Chen, J. M.; Chen, X.; Cho, A.; Chong, L. S.; Desai, M.; Fardis, M.; Kirschberg, T.; Mackman, R. L.; Swaminathan, S.; Watkins, W. J. US 7417055B2, 2004[Chem. Abstr. 2004, 149, 307869].
-
[19]
Yan, W.; Stone, E.; Zhang, Y. Biochemistry 2017, 56, 876. doi: 10.1021/acs.biochem.6b01172
-
[20]
Studer, A.; Seebach, D. Heterocycles 1995, 40, 357. doi: 10.3987/COM-94-S44
-
[21]
Katritzky, A. R.; Suzuki, K.; He, H. J. Org. Chem. 2002, 67, 3109. doi: 10.1021/jo010868n
-
[22]
Schickll, C. P.; Seebach, D. Liebigs Ann. Chem. 1991, 655.
-
[23]
Pudovik, A. N.; Shagidullin, R. R.; Khairullin, V. K.; Vandyukova, I. I.; Chernova, A. V.; Gainullin, R. M.; Pudovik, M. A. Izv. Akad. Nauk, Ser. Khim. 1996, 1303.
-
[24]
Bailly, T.; Burgada, R. Phosphorus. Sulfur. Silicon. Relat. Elem. 1995, 101, 131. doi: 10.1080/10426509508042509
-
[25]
Viveros-Ceballos, J.; Ordonez, M.; Sayago, F. J.; Jimenez, A. I.; Cativiela, C. Eur. J. Org. Chem. 2016, 2711.
-
[1]
-
表 1 1a与2a反应的条件筛选a
Table 1. Condition screening for the reaction of 1a with 2a

Entry Base Solvent Time/h Yieldb/% 1 LiOH EtOAc 9 41 2 K2CO3 EtOAc 9 <5 3 NaOH EtOAc 9 <5 4 KOH EtOAc 9 0 5b LiOH EtOAc 9 41 6c LiOH EtOAc 9 39 7d LiOH EtOAc 9 39 8 LiOH DMF 9 15 9 LiOH CH3CN 9 13 10 LiOH CH3COCH3 9 25 11 LiOH THF 9 32 12 LiOH DCM 9 14 13 LiOH DCE 9 27 14 LiOH Et2O 9 30 15 LiOH PhCH3 9 36 16 LiOH n-Hexane 9 47 17 LiOH Petroleum ether 9 50 18e LiOH Petroleum ether 9 9 19f LiOH Petroleum ether 9 20 20g LiOH Petroleum ether 5 83 a Reaction conditions: base (2 mmol), 1a (1 mmol), 2a (0.2 mmol), solvent (3 mL), at room temperature under Ar; isolated yield after column chromatography. b 2a (0.14 mmol); c 2a (0.25 mmol); d 2a (0.33 mmol); eat 50 ℃; f at 0 ℃; g 3a (0.25 mmol) was used instead of 2a. 表 2 苯磺砜1与3反应合成4 a
Table 2. Preparation of 4 by the reaction of phenylsulfones 1 with 3



aReaction conditions: LiOH (2 mmol), 1 (1 mmol), 3 (0.25 mmol), petroleum ether (3 mL), at room temperature under Ar; isolated yield after column chromatography. 表 3 对甲苯磺砜1与3反应合成4a
Table 3. Preparation of 4 by the reaction of tolylsulfones 1 with 3






a Reaction conditions: LiOH (2 mmol), 1 (1 mmol), 3 (0.25 mmol), petroleum ether (3 mL), at room temperature under Ar; isolated yield after column chromatography. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 4
- 文章访问数: 1105
- HTML全文浏览量: 193


 下载:
下载:
 下载:
下载:

