 图 1
含4-喹啉酮骨架的抗菌药物
Figure 1.
Antimicrobial agent with 4-quinolinone skeleton
图 1
含4-喹啉酮骨架的抗菌药物
Figure 1.
Antimicrobial agent with 4-quinolinone skeleton

Citation: Chen Xuebing, Bai Hairui, Huang Chao. Concise Synthesis of Quinolinone Derivatives[J]. Chinese Journal of Organic Chemistry, 2017, 37(4): 881-888. doi: 10.6023/cjoc201611003

喹啉酮类化合物的简便合成
English
Concise Synthesis of Quinolinone Derivatives
-
Key words:
- β-enaminones
- / regionselectivity acylation
- / intramolecular cyclization
- / quinolinones
-
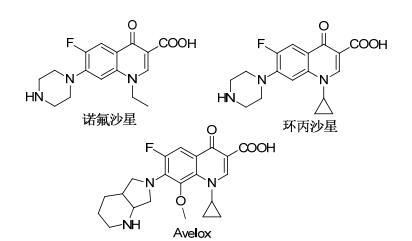
喹啉酮及其衍生物是一类重要的杂环化合物, 其骨架广泛存在于天然产物或者药物活性分子中[1].在所有的喹啉类化合物中, 4-喹啉酮以其显著的生物活性及其在药物中的大量应用而成为人们研究的热点, 其生物活性主要包括抗病毒[2]、抗HIV[3]、抗菌[4]、抗肿瘤[5], 以及作为DNA旋转酶和拓扑异构酶Ⅱ抑制剂[6]和大麻素2型受体激动剂[7]等. 4-喹啉酮母体结构在天然药物和化学合成药物中都很常见[8], 尤其是抗菌药物.自1962年第一个喹啉酮类抗菌药物萘啶酸面世以来, 以4-喹啉酮为先导化合物开发出来的抗菌药物越来越多, 其中最具代表性的包括诺氟沙星、环丙沙星、Levaquin、Vigamox、Avelox (Figure 1)[4a, b].此外, 4-喹啉酮因为含有独特的烯胺酮结构, 使得其成为一个重要的有机合成砌块, 并广泛应用于药物中间体合成[9].鉴于4-喹啉酮在生物医药和有机合成中的重要作用, 近年来关于该类化合物的合成报道很多, 主要方法包括过渡金属催化的碳-氮键偶联反应[10]和分子内加成缩合反应[11].上述方法在合成中往往分子多样性很难得到体现, 且许多方法反应条件苛刻, 得到的化合物可修饰性较弱.因此有必要继续探究新的合成4-喹啉酮类化合物的方法.
烯胺酮是一类重要的有机合成中间体, 含有独特的氮杂烯结构, 已被广泛用于合成结构多样性的杂环化合物[12].这些杂环化合物包括吲哚[13]、吖啶[14]、喹啉[15]、萘啶[16]、吡啶[17]、吡咯[18], 其中部分化合物表现出一定的生物活性[19].然而基于传统简单结构烯胺酮合成出来的杂环化合物在生物活性测试中表现不够优异, 且产物可修饰位点较少, 在药物化学中应用存在不足, 本文设计从β-烯胺酮出发, 通过与氰基乙酸的酰基化及分子内环合反应, 得到一类2-氨基喹啉酮类化合物 (Scheme 1).此类化合物保留了传统烯胺酮化合物的所有反应位点, 且在2-位引入了一个可修饰性较强的氨基, 将大大扩充烯胺的反应及其产物的骨架丰富性, 有助于发现新的有机反应和先导化合物.
1 结果与讨论
1.1 氰基乙酰基烯胺酮3的制备
烯胺酮结构中含有氮杂烯H—N—C=C结构, 因此其α-C具有较强的亲核性.根据类似文献报道[20], 氰基乙酸在乙酸酐作用下可以得到氰基乙酸乙酯, 进而与烯胺酮通过亲核取代反应得到3-氰基乙酰基烯胺酮类化合物.本文从不同取代的烯胺酮1和氰基乙酸 (2) 出发, 在醋酸酐作用下, 制备得到了20个分子多样性的3-氰基乙酰基烯胺酮3a~3t (Eq. 1).
1.2 喹啉酮类化合物4的制备
1.2.1 反应条件筛选
得到中间体3-氰基乙酰基烯胺酮3以后, 开始尝试通过其分子内环合来构建目标产物喹啉酮类化合物4.我们选取中间体3f进行模板反应, 通过对反应溶剂、催化剂、时间、温度等条件的筛选来确定最优反应条件, 结果如表 1所示.

Entry Solvent Catalyst T/℃ t/h Yieldb/% 1 EtOH — r.t. 4 N.R. 2 EtOH — Reflux 4 N.R. 3 EtOH Piperidine r.t. 2 63 4 EtOH Piperidine 40 1 86 5 EtOH Et3N 40 1 84 6 EtOH DBU 40 1 76 7 EtOH PPh3 40 4 N.R. 8 EtOH HOAc 40 1 N.R. 9 EtOH p-TSA 40 1 N.R. 10 EtOH L-Proline 40 1 72 11 Acetonitrile Piperidine 40 1 91 12 1, 4-Dioxane Piperidine 40 1 86 13 CH2Cl2 Piperidine 40 1 86 14 CH3OH Piperidine 40 1 78 15 H2O Piperidine 40 4 Trace a The reaction was performed with 3f (1.0 mmol), catalyst (0.3 mmol), and solvent (10 mL); b Isolated yield; N.R.=no reaction. 表 1 反应条件的优a
Table 1. Optimization of reaction conditions首先, 反应以乙醇作为溶剂, 在无催化剂条件下进行, 结果无论在室温还是回流条件下4 h, 此反应都不能进行 (Table 1, Entries 1~2).然而, 当0.3 mmol哌啶加入到室温下反应液中时, 化合物4a以63%的收率得到 (Table 1, Entry 3);进一步调节温度发现, 40 ℃时反应可以快速完成, 并且产率达到86% (Table 1, Entry 4).为了进一步优化反应条件, 接着考察了其它催化剂, 包括三乙胺、DBU、三苯基膦、醋酸、对甲基苯磺酸、L-脯氨酸, 结果显示, 催化剂对反应结果影响较大, 碱性催化剂较酸性催化剂对反应更有利, 尤其是醋酸不能催化该反应 (Table 1, Entries 5~10).上述结果表明, 哌啶为该反应的最佳催化剂.最后, 对反应溶剂进行了筛选.质子性溶剂甲醇、水和非质子性溶剂乙腈、1, 4-二氧六环、二氯甲烷被用于该反应, 结果表明乙腈给出了比乙醇更高的产率, 产率高达91% (Table 1, Entries 11~15).最终确定了反应的最佳条件为哌啶为催化剂, 乙腈做溶剂, 反应温度为40 ℃, 反应时间为1 h.
1.2.2 反应底物扩充
在得到最优反应条件以后, 我们对底物进行了扩充.通过改变氰基乙酰基烯胺酮2环己基5-位的取代基或者苯环上的取代基, 得到了一类分子多样性的喹啉酮类化合物4a~4t (Table 2).如表 2所示, 氰基乙酰基烯胺酮2的4位碳上不管是氢还是双甲基取代, 目标产物都可以以较高产率得到; 同样地, 氰基乙酰基烯胺酮2的芳环上不管有供电子的取代基甲基、甲氧基或者吸电子的卤素取代基以及取代位置的不同, 均可以得到目标产物4.值得注意的是, 所有化合物均不需要经过柱层析, 而只需简单的过滤、洗涤、重结晶.

Entry R R1 3 4 Yieldb/% 1 4-Fluorophenyl H 3a 4a 87 2 3-Fluorophenyl H 3b 4b 89 3 4-Chlorophenyl H 3c 4c 86 4 4-Bromophenyl H 3d 4d 95 5 3-Bromophenyl H 3e 4e 92 6 Phenyl H 3f 4f 91 7 4-Methylphenyl H 3g 4g 87 8 3-Methylphenyl H 3h 4h 90 9 4-Methoxyphenyl H 3i 4i 91 10 4-Fluorophenyl CH3 3j 4j 93 11 4-Chloro phenyl CH3 3k 4k 91 12 3-Chlorophenyl CH3 3l 4l 84 13 2-Chlorophenyl CH3 3m 4m 90 14 4-Bromophenyl CH3 3n 4n 94 15 3-Bromophenyl CH3 3o 4o 90 16 Phenyl CH3 3p 4p 87 17 4-Methylphenyl CH3 3q 4q 89 18 3-Methylphenyl CH3 3r 4r 86 19 2-Methylphenyl CH3 3s 4s 82 20 4-Methoxyphenyl CH3 3t 4t 90 a The reaction was performed with 3 (1.0 mmol), catalyst (0.3 mmol), and solvent (10 mL); b Isolated yield. 表 2 目标化合物4的合成
Table 2. Synthesis of target molecules 41.2.3 反应机理
我们以化合物4p为例, 对可能的反应机理进行阐述 (Scheme 2).在碱的催化下, 中间体3p的氨基对氰基进行亲核加成得到中间体5, 中间体5通过亚胺-烯胺互变得到目标产物4p.
2 结论
本文以简单易得合成子β-烯胺酮为砌块, 和氰基乙酸在醋酸酐作用下, 得到了一类氰乙酰基化的烯胺酮化合物.进而在哌啶催化下, 乙腈为溶剂, 实现了上述化合物的分子内环合反应, 成功合成了一类结构新颖且具有潜在生物活性的喹啉酮类杂环化合物.此类化合物是对烯胺酮骨架的扩充, 且因母核含有喹啉酮结构单元, 以此为砌块合成的杂环化合物将具备更丰富的官能团信息, 并为发现新的先导化合物提供化合物基础.此外, 该方法还具有操作简便, 反应产率高, 后处理简单等优点.
3 实验部分
3.1 仪器与试剂
反应使用控温电磁搅拌, 在烘干玻璃的仪器中进行; XT4A型显微熔点测定仪 (控温型) 测定熔点; 使用Thermo Nicolet Avatar360型傅立叶红外光谱仪测定IR, 均采用KBr压片法; 使用Agllent CL/Msd TOF质谱仪测定MS; 使用Bruck DRX 400型核磁共振仪测定NMR, TMS作为内标, 氘代二甲亚砜和高氯酸 (增加目标产物溶解性) 作为溶剂.
实验所涉及试剂均为化学纯或分析纯, 溶剂用无水硫酸钠或分子筛干燥处理; 柱层析使用硅胶为青岛海洋化工 (200~300目), 如果不做特别说明, 流动相均采用重蒸工业级石油醚和乙酸乙酯; 反应监控用薄层色谱 (TLC) 方法, 青岛海洋化工厂生产的薄层层析板 (GF254高效板).
3.2 实验方法
3.2.1 氰乙酰基烯胺酮化合物3的合成
在25 mL的圆底烧瓶中, 分别加入烯胺酮 (1 mmol)、氰基乙酸 (1 mmol)、乙酸酐 (5 mL), 加热于50 ℃, TLC监测反应完成, 冷却至室温, 饱和碳酸钠溶液中和, 用乙酸乙酯 (50 mL) 萃取两次, 无水Na2SO4干燥, 减压蒸馏浓缩, 柱层析分离得到粗产物, 乙酸乙酯/石油醚重结晶得到化合物3.因合成类似化合物文献较多, 本文仅选取其中代表性化合物3q进行结构表征, 数据如下:
3-[4, 4-二甲基-6-氧代-2-(对甲苯氨基) 环己烷-1-烯-1-基]-3-氧代丙腈 (3q):浅黄色固体, m.p. 105~106 ℃, 收率83%. 1H NMR (CDCl3, 400 MHz) δ: 13.61 (br, 1H, NH), 7.23 (d, J=8.0 Hz, 2H, ArH), 7.00 (d, J=8.0 Hz, 2H, ArH), 4.24 (s, 2H, CH2), 2.44 (s, 2H, CH2), 2.37 (s, 3H, CH3), 2.31 (s, 2H, CH2), 0.98 (s, 6H, 2×CH3); 13C NMR (100 MHz, 400 MHz, CDCl3) δ: 194.9, 188.9, 172.5, 138.5, 133.5, 130.4, 125.7, 115.8, 106.7, 51.9, 41.8, 34.2, 31.2, 27.9, 21.2; HRMS (ESI-TOF) calcd for C18H21N2O2 (M+H)+ 297.1598, found 297.1593.
3.2.2 双环吡啶类化合物4的合成通法
取1.0 mmol氰乙酰基烯胺酮3于25 mL圆底烧瓶中, 加入15 mL无水乙腈和0.30 mmol哌啶, 40 ℃搅拌反应约1 h, TLC监测底物3反应完全, 待反应液冷却至室温, 抽滤, 用冰乙醇 (5 mL) 洗涤固体三次得到纯品喹啉酮类化合物4, 产率为82%~95%.所有化合物的结构都经过核磁共振、红外以及高分辨质谱表征.
2-氨基-1-(4-氟苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4a): 238 mg, 产率87%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 7.57~7.63 (m, 1H, ArH), 7.37~7.40 (m, 1H, ArH), 7.27~7.32 (m, 1H, ArH), 7.53~7.57 (m, 1H, ArH), 6.26 (s, 1H, CH), 2.54~ 2.61 (m, 2H, CH2), 2.40~2.45 (m, 2H, CH2), 1.93~1.98 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 203.3, 168.7, 163.5 (d, 1JC—F=246.9 Hz), 165.0, 162.9, 158.0, 130.7, 130.6, 119.0 (d, 2JC—F=23.1 Hz), 118.7 (d, 2 JC—F=23.1 Hz), 109.4, 93.7, 36.2, 29.0, 20.1; IR (KBr) ν: 3453, 1661, 1638, 1440, 1224, 995, 847 cm-1; HRMS (ESI-TOF) calcd for C15H14FN2O2 (M+H) + 273.1034, found 273.1035.
2-氨基-1-(3-氟苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4b): 243 mg, 产率89%.白色固体, m.p. 283~284 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 13.00 (br, 1H, NH2), 7.79~7.83 (m, 2H, ArH), 7.57~7.61 (m, 2H, ArH), 7.44~7.47 (m, 1H, ArH), 6.39 (s, 1H, CH), 2.61~2.64 (m, 2H, CH2), 2.51~2.53 (m, 2H, CH2), 1.95~1.98 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.8, 168.6, 163.5 (d, J=246.0 Hz), 162.6, 157.8, 135.7, 133.6 (d, J=9.0 Hz), 124.6, 119.3 (d, J=21.0 Hz), 116.2 (d, J=25.0 Hz), 109.3, 93.9, 36.5, 29.0, 20.3; IR (KBr) ν: 3479, 1671, 1639, 1512, 1438, 1295, 806 cm-1; HRMS (ESI-TOF) calcd for C15H14FN2O2 (M+H) + 273.1034, found 273.1038.
2-氨基-1-(4-氯苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4c): 249 mg, 产率86%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 13.03 (br, 1H, NH2), 7.81~7.84 (m, 2H, ArH), 7.61~7.64 (m, 2H, ArH), 6.34 (s, 1H, CH), 2.61~2.64 (m, 2H, CH2), 2.44~2.48 (m, 2H, CH2), 1.96~1.99 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 203.0, 168.7, 162.7, 157.9, 136.8, 133.5, 132.0, 130.2, 109.4, 93.8, 36.4, 29.1, 20.3; IR (KBr) ν: 3469, 1674, 1635, 1455, 1379, 1289, 845 cm-1; HRMS (ESI-TOF) calcd for C15H14ClN2O2 (M + H) + 289.0738, found 289.0748.
2-氨基-1-(4-溴苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4d): 306 mg, 产率95%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 12.87 (br, 1H, NH2), 7.95 (d, J=8.0 Hz, 2H, ArH), 7.56 (d, J=8.0 Hz, 2H, ArH), 6.40 (s, 1H, CH), 2.61~2.64 (m, 2H, CH2), 2.45~2.48 (m, 2H, CH2), 1.96~1.99 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 203.0, 168.6, 162.6, 157.9, 134.9, 133.9, 130.4, 125.6, 109.4, 93.8, 36.4, 29.1, 20.3; IR (KBr) ν: 3433, 1680, 1642, 1453, 1293, 1187, 847 cm-1; HRMS (ESI-TOF) calcd for C15H14BrN2O2 (M+H)+ 333.0233, found 333.0236.
2-氨基-1-(3-溴苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4e): 306 mg, 产率92%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 13.02 (br, 1H, NH2), 7.77~7.80 (m, 2H, ArH), 7.67~7.70 (m, 1H, ArH), 7.61~7.63 (m, 1H, ArH), 6.37 (s, 1H, CH), 2.61~2.64 (m, 2H, CH2), 2.45~2.48 (m, 2H, CH2), 1.96~1.99 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.8, 168.6, 162.6, 157.9, 135.8, 135.1, 133.5, 131.2, 127.6, 123.9, 109.3, 93.9, 36.5, 29.1, 20.3; IR (KBr) ν: 3447, 1682, 1640, 1420, 1302, 1261, 883 cm-1; HRMS (ESI-TOF) calcd for C15H14BrN2O2 (M+H)+ 333.0233, found 333.0234.
2-氨基-1-苯基-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4f): 232 mg, 产率91%.白色固体, m.p. 275~277 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 7.78~7.84 (m, 3H, ArH), 7.52~7.56 (m, 2H, ArH), 6.30 (s, 1H, CH), 2.58~2.62 (m, 2H, CH2), 2.40~2.44 (m, 2H, CH2), 1.94~1.98 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d6 +HClO4) δ: 203.3, 168.7, 162.7, 157.8, 134.6, 132.0, 131.8, 128.0, 109.3, 93.7, 36.3, 29.0, 20.2; IR (KBr) ν: 3471, 1663, 1639, 1436, 1287, 1228, 704 cm-1; HRMS (ESI-TOF) calcd for C15H15N2O2 (M+H) + 255.1128, found 255.1128.
2-氨基-1-(4-甲苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4g): 234 mg, 产率87%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 8.49(d, J=8.0 Hz, 2H, ArH), 8.37 (d, J=8.0 Hz, 2H, ArH), 7.23 (s, 1H, CH), 3.54~3.58 (m, 2H, CH2), 3.37~3.41 (m, 5H, CH2+ CH3), 2.90~2.94 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 203.4, 168.7, 162.9, 157.9, 141.8, 132.5, 127.7, 109.3, 93.7, 36.2, 28.9, 21.3, 20.1; IR (KBr) ν: 3471, 1663, 1651, 1453, 1397, 1191, 838 cm-1; HRMS (ESI-TOF) calcd for C16H17N2O2 (M+H) + 269.1285, found 269.1288.
2-氨基-1-(3-甲苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4h): 242 mg, 产率90%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 13.03 (br, 1H, NH2), 9.16 (br, 1H, NH2), 7.61 (d, J=8.0 Hz, 1H, ArH), 7.53 (d, J=8.0 Hz, 1H, ArH), 7.37 (t, J=8.0 Hz, 2H, ArH), 6.43 (s, 1H, CH), 2.61~2.64 (m, 2H, CH2), 2.46~ 2.49 (m, 2H, CH2), 2.42 (s, 3H, CH3), 1.95~1.98 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.9, 168.4, 162.7, 157.8, 141.6, 134.5, 132.5, 131.5, 128.3, 125.0, 109.3, 93.8, 36.5, 29.0, 21.4, 20.3; IR (KBr) ν: 3451, 1657, 1639, 1438, 1295, 1183, 826 cm-1; HRMS (ESI-TOF) calcd for C16H17N2O2 (M+H) + 269.1285, found 269.1286.
2-氨基-1-(4-甲氧苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4i): 259 mg, 产率91%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 13.03 (br, 1H, NH2), 7.46~7.50 (m, 2H, ArH), 7.25~7.28 (m, 2H, ArH), 6.33 (s, 1H, CH), 3.87 (s, 3H, OCH), 2.60~2.64 (m, 2H, CH2), 2.47~2.51 (m, 2H, CH2), 1.94~1.98 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 203.1, 168.5, 163.4, 161.4, 158.3, 129.4, 127.0, 116.9, 109.3, 93.6, 56.2, 36.4, 29.1, 20.3; IR (KBr) ν: 3395, 1665, 1642, 1508, 1300, 1251, 843 cm - 1; HRMS (ESI-TOF) calcd for C16H17N2O3 (M+H)+ 285.1234, found 285.1235.
2-氨基-7, 7-二甲基-1-(4-氟苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4j): 280 mg, 产率93%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 7.60~7.64 (m, 2H, ArH), 7.53~7.57 (m, 2H, ArH), 6.30 (s, 1H, CH), 5.11 (br, 2H, NH2), 2.53 (s, 2H, CH2), 2.37 (s, 2H, CH2), 0.94 (s, 6H, 2×CH3); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.7, 168.3, 163.8 (d, 1JC—F= 247.1 Hz), 160.7, 158.4, 130.7, 130.6 (d, 3JC—F=4.8 Hz), 119.1 (d, 2J C—F=23.3 Hz), 118.9 (d, 2J C—F=23.3 Hz), 108.6, 94.0, 49.5, 41.8, 32.7, 27.8; IR (KBr) ν: 3451, 1671, 1639, 1506, 1216, 1148, 846 cm-1; HRMS (ESI-TOF) calcd for C17H18FN2O2 [(M + H) +] 301.1347, found 301.1348.
2-氨基-7, 7-二甲基-1-(4-氯苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4k): 288 mg, 产率91%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 7.57 (d, J=8.0 Hz, 2H, ArH), 7.36 (d, J=8.4 Hz, 2H, ArH), 6.05 (s, 1H, CH), 5.91 (br, 2H, NH2), 2.25~2.31 (m, 3H, CH2), 2.11~2.14 (m, 1H, CH2), 0.70 (s, 6H, 2× CH3); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.4, 168.3, 160.5, 158.3, 136.8, 133.4, 132.1, 130.2, 108.6, 94.0, 49.6, 41.8, 32.7, 27.7; IR (KBr) ν: 3457, 1653, 1647, 1432, 1306, 1109, 848 cm-1; HRMS (ESI-TOF) calcd for C17H18ClN2O2 (M+H)+ 317.1051, found 317.1054.
2-氨基-7, 7-二甲基-1-(3-氯苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4l): 266 mg, 白色固体, 产率84%. m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 12.87 (br, 1H, NH2), 7.75~7.82 (m, 3H, ArH), 7.57~7.59 (m, 1H, ArH), 6.35 (s, 1H, CH), 2.55 (s, 2H, CH2), 2.43 (AB, J=16.0 Hz, 1H, CH2), 2.36 (AB, J=16.0 Hz, 1H, CH2), 0.97 (s, 3H, CH3), 0.96 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.1, 168.2, 160.4, 158.2, 135.8, 133.5, 132.3, 128.5, 127.2, 108.6, 94.1, 49.7, 40.6, 32.8, 27.8, 27.7; IR (KBr) ν: 3446, 1665, 1645, 1446, 1287, 1102, 851 cm - 1; HRMS (ESI-TOF) calcd for C16H17N2O2 (M+H)+ 317.1051, found 317.1058.
2-氨基-7, 7-二甲基-1-(2-氯苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4m): 285 mg, 产率90%.白色固体, m.p. 290~291 ℃; 1H NMR (400 MHz, DMSO-d6+ HClO4) δ: 12.90 (br, 1H, NH2), 7.92~7.94 (m, 1H, ArH), 7.72~7.82 (m, 3H, ArH), 6.42 (s, 1H, CH), 2.58 (s, 2H, CH2), 2.52~2.65 (m, 3H, CH2), 2.07~2.12 (m, 1H, CH2), 0.98 (s, 3H, CH3), 0.95 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 201.7, 168.5, 160.1, 157.6, 134.2, 132.4, 131.6, 131.0, 131.0, 130.5, 109.0, 94.1, 49.7, 41.2, 32.7, 28.5, 26.9; IR (KBr) ν: 3449, 1663, 1640, 1435, 1304, 1234, 769 cm - 1; HRMS (ESI-TOF) calcd for C17H18ClN2O2 (M+H)+ 317.1051, found 317.1053.
2-氨基-7, 7-二甲基-1-(4-溴苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4n): 339 mg, 产率94%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 12.85 (br, 1H, NH2), 7.97 (d, J=8.0 Hz, 1H, ArH), 7.55 (d, J=8.0 Hz, 2H, ArH), 6.34 (s, 1H, CH), 2.37~2.41 (m, 2H, CH2), 2.53~2.57 (m, 2H, CH2), 0.97 (s, 6H, 2×CH3); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.3, 168.2, 160.5, 158.2, 135.1, 133.9, 130.4, 125.6, 108.7, 94.1, 49.3, 41.8, 32.8, 27.8; IR (KBr) ν: 3445, 1667, 1610, 1544, 1445, 1290, 849 cm - 1; HRMS (ESI-TOF) calcd for C17H18BrN2O2 (M+H)+ 361.0546, found 361.0548.
2-氨基-7, 7-二甲基-1-(3-溴苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4o):得325 mg, 产率90%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 12.86 (br, 1H, NH2), 7.93~7.97 (m, 2H, ArH), 7.69~ 7.73(m, 1H, ArH), 7.61~7.63 (m, 1H, ArH), 6.35 (s, 1H, CH), 2.51~2.55 (m, 2H, CH2), 2.43 (AB, J=8.0 Hz, 1H, CH2), 2.36 (AB, J=8.0 Hz, 1H, CH2); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.1, 168.2, 160.4, 158.2, 135.8, 135.2, 133.7, 131.1, 127.6, 124.1, 108.6, 94.1, 49.7, 40.4, 32.8, 27.9, 27.7; IR (KBr) ν: 3447, 1665, 1616, 1402, 1294, 1102, 712 cm - 1; HRMS (ESI-TOF) calcd for C17H18BrN2O2 (M+H)+ 361.0546, found 361.0544.
2-氨基-7, 7-二甲基-1-苯基-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4p): 246 mg, 产率87%.白色固体, m.p. 288~ 289 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 12.89 (br, 1H, NH2), 7.71~7.78 (m, 3H, ArH), 7.57 (d, J=8.0 Hz, 2H, ArH), 6.40 (s, 1H, CH), 2.52~2.56 (m, 2H, CH2), 2.36 (s, 2H, CH2), 0.95 (s, 6H, 2×CH3); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.3, 168.2, 160.6, 158.3, 134.6, 132.0, 128.1, 108.6, 94.1, 49.7, 41.9, 32.7, 27.8; IR (KBr) ν: 3447, 1670, 1638, 1430, 1289, 1053, 840 cm-1; HRMS (ESI-TOF) calcd for C17H19N2O2 (M + H) + 283.1441, found 283.1445.
2-氨基-7, 7-二甲基-1-(4-甲苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4q):得264 mg, 产率89%.白色固体, m.p. 295~297 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 7.57 (d, J=8.0 Hz, 2H, ArH), 7.36 (d, J=8.4 Hz, 2H, ArH), 6.05 (s, 1H, CH), 5.91 (br, 2H, NH2), 2.25~2.31 (m, 3H, CH2), 2.11~2.14 (m, 1H, CH2), 0.70 (s, 6H, 2× CH3); 13C NMR (100 MHz, DMSO-d6) δ: 194.3, 174.6, 156.4, 153.0, 140.1, 133.8, 131.5, 129.0, 116.9, 96.4, 52.2, 42.6, 31.9, 28.2, 21.3; IR (KBr) ν: 3451, 1702, 1642, 1434, 1297, 1053, 820 cm - 1; HRMS (ESI-TOF) calcd for C18H21N2O2 (M+H)+ 297.1598, found 297.1599.
2-氨基-7, 7-二甲基-1-(3-甲苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4r): 255 mg, 产率86%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 12.87 (br, 1H, NH2), 7.64 (t, J=8.0 Hz, 1H, ArH), 7.54 (d, J=8.0 Hz, 1H, ArH), 7.37 (d, J=8.0 Hz, 2H, ArH), 6.36 (s, 1H, CH), 2.51~2.56 (m, 2H, CH2), 2.43 (s, 3H, CH3), 2.37~2.41 (m, 2H, CH2), 0.96 (s, 3H, CH3), 0.95 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.3, 168.2, 160.6, 158.2, 141.8, 134.5, 132.7, 131.7, 128.2, 125.0, 108.6, 94.0, 40.4, 32.8, 27.9, 27.7, 21.5; IR (KBr) ν: 3453, 1667, 1635, 1430, 1402, 1283, 836 cm-1; HRMS (ESI-TOF) calcd for C18H21N2O2 (M + H) + 297.1598, found 297.1603.
2-氨基-7, 7-二甲基-1-(2-甲苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4s): 244 mg, 产率82%.白色固体, m.p. 280~282 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 7.62~7.67 (m, 2H, ArH), 7.55~7.59 (m, 1H, ArH), 7.48 (d, J=8.0 Hz, 1H, ArH), 6.49 (s, 1H, CH), 2.58 (s, 2H, CH2), 2.48~2.51 (m, 1H, CH2), 2.12~2.15 (m, 1H, CH2), 2.07 (s, 3H, CH3), 0.97 (s, 3H, CH3), 0.96 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.2, 168.4, 160.1, 157.6, 135.2, 133.3, 133.3, 129.6, 128.8, 109.0, 94.1, 49.6, 41.3, 39.7, 27.7, 26.7, 16.7; IR (KBr) ν: 3440, 1663, 1616, 1438, 1285, 1242, 724 cm - 1; HRMS (ESI-TOF) calcd for C18H21N2O2 (M+H) + 297.1598, found 297.1598.
2-氨基-7, 7-二甲基-1-(4-甲氧苯基)-7, 8-二氢喹啉-4, 5(1H, 6H)-二酮 (4t): 282 mg, 产率90%.白色固体, m.p.>300 ℃; 1H NMR (400 MHz, DMSO-d6+HClO4) δ: 7.44 (d, J=7.6 Hz, 2H, ArH), 7.23 (d, J=7.6 Hz, 2H, ArH), 6.27 (s, 1H, CH), 5.28 (br, 2H, NH2), 3.85 (s, 3H, OCH3), 2.52 (s, 2H, CH2), 2.38 (s, 2H, CH2), 0.93 (s, 6H, 2×CH3); 13C NMR (100 MHz, DMSO-d6+HClO4) δ: 202.9, 168.3, 161.3, 161.1, 158.6, 129.3, 126.8, 116.9, 108.5, 93.8, 56.1, 49.4, 41.8, 32.6, 27.8; IR (KBr) ν: 3481, 1678, 1631, 1514, 1426, 1218, 855 cm-1; HRMS (ESITOF) calcd for C18H21N2O3 (M+H)+ 313.1547, found 313.1546.
辅助材料 (Supporting Information) 化合物3p, 4a~4t的1H NMR和13C NMR图谱.这些材料可以免费从本刊网 (http://sioc-journal.cn/) 上下载.
-
-
[1]
(a) Cho, J.-Y.; Bae, S.-H.; Kim, H.-K.; Lee, M.-Y.; Choi, Y.-S.; Jin, B.-R.; Lee, H.-J.; Jeong, H.-Y.; Lee, Y.-G.; Moon, J.-K. J. Agric. Food Chem. 2015, 63, 3587.
(b) He, J.; Lion, U.; Sattler, S.; Gollmick, F. A.; Grabley, S.; Cai, J.; Meiners, M.; Schünke, H.; Schaumann, K.; Dechert, U.; Krohn, M. J. Nat. Prod. 2005, 68, 1397.
(c) Freeman, G. A.; Andrews Ⅲ, C. W.; Hopkins, A. L.; Lowell, G. S.; Schaller, L. T.; Cowan, J. R.; Gonzales, S. S.; Koszalka, G. W.; Hazen, R. J.; Boone, L. R.; Ferris, R. G.; Creech, K. L.; Roberts, G. B.; Short, S. A.; Weaver, K.; Reynolds, D. J.; Milton, J.; Ren, J.; Stuart, D. I.; Stammers, D. K.; Chan, J. H. J. Med. Chem. 2004, 47, 5923.
(d) Godard, A.; Fourquez, J. M.; Tamion, R.; Marsais, F.; Quéguiner, G. Synlett 1994, 235.
(e) Goossen, L. J.; Deng, G.; Levy, L. M. Science 2006, 313, 662.
(f) Nakatsu, T.; Johns, T.; Kubo, I.; Milton, K.; Sakai, M.; Chatani, K.; Saito, K.; Yamagiwa, Y.; Kamikawa, K. J. Nat. Prod. 1990, 53, 1508. -
[2]
(a) Linás, B. M.; Bailey, M. D.; Ghiro, E.; Gorys, V.; Halmos, T.; Poirier, M.; Rancourt, J.; Goudreau, N. J. Med. Chem. 2004, 47, 6584.
(b) Lucero, B. A.; Gomes, C. R. B.; Frugulhetti, I. C. P. P.; Faro, L. V.; Alvarenga, L.; Souza, M. C. B.V.; de Souza, T. M. L.; Ferreira. V. F. Bioorg. Med. Chem. Lett. 2006, 16, 1010. -
[3]
(a) Sato, M.; Motomura, T.; Aramaki, H.; Matsuda, T.; Yamashita, M.; Ito, Y.; Kawakami, H.; Matsuzaki, Y.; Watanabe, W.; Yamataka, K.; Ikeda, S.; Kodama, E.; Matsuoka, M.; Shinkai, H. J. Med. Chem. 2006, 49, 1506.
(b) Santo, R. D.; Costi, R.; Roux, A.; Artico, M.; Lavecchia, A.; Marinelli, L.; Novellino, E.; Palmisano, L.; Andreotti, M.; Amici, R.; Galluzzo, C. M.; Nencioni, L.; Palamara, A. T.; Pommier, Y.; Marchand, C. J. Med. Chem. 2006, 49, 1939. -
[4]
(a) Vahaboglu, H.; Budak, F.; Kasap, M.; Gacar, G.; Torol, S.; Karadenizli, A.; Kolayli, F.; Eroglu, C. J. Antimicrob. Chemother. 2006, 58, 537.
(b) Chai, Y.; Wan, Z.-L.; Wang, B.; Guo, H.-Y.; Liu, M.-L. Eur. J. Med. Chem. 2009, 44, 4063.
(c) Yang, J.-Q.; Hu, Y.-W.; Gu, Q.; Li, M.-G.; Li, M.-Q.; Song, B.-A. Chin. J. Org. Chem. 2014, 34, 829 (in Chinese). (杨家强, 胡月维, 谷晴, 李明刚, 李明强, 宋宝安, 有机化学, 2014, 34, 829.) -
[5]
(a) Xia, Y.; Yang, Z.-Y.; Xia, P.; Bastow, K. F.; Nakanishi, Y.; Nampoothiri, P.; Hamel, E.; Brossi, A.; Lee, K.-H. Bioorg. Med. Chem. Lett. 2003, 13, 2891.
(b) Nakamura, S.; Kozuka, M.; Bastow, K. F.; Tokuda, H.; Nishino, H.; Suzuki, M.; Tatsuzaki, J.; Natschke, S. L. M.; Kuo, S.-C.; Lee, K.-H. Bioorg. Med. Chem. 2005, 13, 4396.
(c) Messaoudi, S.; Peyrat, J.-F.; Brion, J.-D.; Alami, M. Anticancer Agents Med. Chem. 2008, 8, 761.
(d) Janin, Y. L. Drug Discovery Today 2010, 15, 342. -
[6]
Sui, Z. H.; Nguyen, V. N.; Altom, J.; Fernandez, J.; Hilliard, J. J.; Bernstein, J. I.; Barrett, J. F.; Ohemeng, K. A. Eur. J. Immunol. 1999, 34, 381.
-
[7]
Stern, E; Muccioli, G. G.; Bosier, B.; Hamtiaux, L.; Millet, R.; Poupaert, J. H.; Henichart, J. P.; Depreux, P.; Goossens, J. F.; Lambert, D. M. J. Med. Chem. 2007, 50, 5471. doi: 10.1021/jm070387h
-
[8]
(a) Michael, J. P. Nat. Prod. Rep. 1997, 14, 605.
(b) EMarques, F. F.; Bueno, M. A.; Duarte, P. D.; Silva, L. R. S. P.; Martinelli, A. M.; dos Santos, C. Y.; Severino, R. P.; Brömme, D.; Vieira, P. C.; Corrêa, A. G. Eur. J. Med. Chem. 2012, 54, 10. -
[9]
(a) Reddy, K. H. V.; Brion, J. H.; Messaoudi, S.; Alami, M. J. Org. Chem. 2016, 81, 424.
(b) Yang, Y.; Yan, H.-J.; Chen, C.-F. Org. Lett. 2007, 9, 4991.
(c) Messaoudi, S.; Brion, J. D.; Alami, M. Org. Lett. 2012, 14, 1496.
(d) Hadida, S.; Goor, F. K.; Zhou, J.-L.; Arumugam, V.; McCartney, J.; Hazlewood, A.; Decker, C.; Negulescu, P.; Grootenhuis, P. D. J. J. Med. Chem. 2014, 57, 9776. -
[10]
(a) Zhao, T.-K.; Xu, B. Org. Lett. 2009, 12, 212.
(b) Mitscher, L. A. Chem. Rev. 2005, 105, 559.
(c) Jones, C.; Anderson, K. W.; Buchwald, S. L. J. Org. Chem. 2007, 72, 7968. (d) Yoshino, Y.; Kurahashi, T.; Matsubara, S. J. Am. Chem. Soc. 2009, 131, 7494.
(d) Huang, J.-K.; Chen, Y.; King, A. O.; Dilmeghani, M.; Larsen, R. D.; Faul, M. M. Org. Lett. 2008, 10, 2609.
(e) Haddad, N.; Tan, J.; Farina, V. J. Org. Chem. 2006, 71, 1626. -
[11]
(a) Liu, R.-H.; Wang, X.-L.; Cheng, F.; Li, S.-F.; Xu, K.-P.; Tan, G.-S. Chin. J. Org. Chem. 2016, 36, 2677 (in Chinese). (刘瑞环, 王绪礼, 成飞, 李福双, 徐康平, 谭桂山, 有机化学, 2016, 36, 2677.)
(b) Huang, Z.-Z; Wu, L.-L.; Huang, X. Chin. J. Org. Chem.2000, 20, 88 (in Chinese). (黄志真, 吴露玲, 黄宪祺, 有机化学, 2000, 20, 88.)
(c) Shmidt, M. S.; Perillo, I. A.; Camelli, A.; Fernández, M. A.; Blanco, M. M. Tetrahedron Lett. 2016, 57, 1022.
(d) Zewge, D.; Chen, C.-Y.; Deer, C.; Dormer, P. G.; Hughes, D. L. J. Org. Chem. 2007, 72, 4276. -
[12]
(a) Cao, S.; Jing, Y.-F.; Liu, Y.-Y.; Wan, J.-P. Chin. J. Org. Chem. 2014, 34, 876 (in Chinese). (曹硕, 井艳锋, 刘云云, 万结平, 有机化学, 2014, 34, 876.)
(b) Li, M.; Guo, W.-S.; Wen, L.-R.; Yang, H.-Z. Chin. J. Org. Chem. 2005, 26, 1192 (in Chinese). (李明, 郭维斯, 文丽荣, 杨华铮, 有机化学, 2005, 26, 1192.) -
[13]
(a) Chen, X.-B.; Luo, T.-B.; Gou, G.-Z.; Wang, J.; Liu, W. Asian J. Org. Chem. 2015, 4, 921.
(b) Jiang, B.; Yi, M.-S.; Shi, F.; Tu, S.-J.; Pindi, S.; McDowell, P.; Li, G.-G. Chem. Comm. 2012, 48, 808.
(c) Hu, J.-D.; Cao, C.-P.; Lin, W.; Hu, M.-H.; Huang, Z.-B.; Shi, D.-Q. J. Org. Chem. 2014, 79, 793. -
[14]
(a) Jiang, B.; Wang, X.; Xu, H.-W.; Tu, M.-S.; Tu, S.-J.; Li, G.-G. Org. Lett. 2013, 15, 1540.
(b) Hao, W.-J.; Wang, J.-Q.; Xu, X.-P.; Zhang, S.-L.; Ji, S.-J. J. Org. Chem. 2013, 78, 12362.
(c) Chen, X.-B.; Wang, X.-Q.; Hong, Y.; Liu, W. Asian J. Org. Chem. 2016, 5, 907.
(d) Wang, H.-Y.; Li, L.-L.; Lin, W.; Xu, P.; Huang, Z.-B.; Shi, D.-Q. Org. Lett. 2012, 14, 4598. -
[15]
(a) Li, M.-Y.; Xu, H.-W.; Fan, W.; Ye, Q.; Wang, X.; Jiang, B.; Wang, S.-L.; Tu, S.-J. Tetrahedron 2014, 70, 1004.
(b) Yu, F.-C.; Zhou, B.; Xu, H.; Li, Y.-M.; Lin, J.; Yan, S.-J.; Shen, Y.-H. Tetrahedron 2015, 71, 1036.
(c) Xu, H.; Zhou, B.; Zhou, J.; Shen, Y.-H.; Yu, F.-C.; Lu, L.-L. Chem. Commun. 2016, 52, 8002.
(d) Xu, H.; Zhou, B.; Zhou, J.; Shen, Y.-H.; Lu, L.-L.; Yu, F.-C. RSC Adv. 2016, 6, 73760. -
[16]
(a) Li, J.; Yu, Y.; Tu, M.-S.; Jiang, B.; Wang, S.-L.; Li, G.-G. Org. Biomol. Chem. 2012, 10, 5361.
(b) Cao, C.-P.; Lin, W.; Hu, M.-H.; Huang, Z.-B.; Shi, D.-Q. Chem. Commun. 2013, 49, 6983. -
[17]
(a) Ma, N.; Jiang, B.; Zhang, G.; Tu, S.-J.; Waver, W.; Li, G.-G. Green Chem. 2010, 12, 1357.
(b) Rana, S.; Brownb, M.; Mukhopadhyay, C. RSC Adv. 2013, 3, 3291. (c) Yu, F.-C.; Zhou, B.; Xu, H.; Chang, K.-J.; Shen, Y.-H. Tetrahedron Lett. 2015, 56, 837.
(c) Yan, S.-J.; Lin, J. Chin. J. Org. Chem. 2010, 30, 465 (in Chinese). (严胜骄, 林军, 有机化学, 2010, 30, 465.)
(d) Peng, M.-Y.; Yang, R.-X.; Liu, X.-M.; Yan, S.-J.; Lin, J. Chin. J. Org. Chem. 2015, 35, 1754 (in Chinese) (彭美阳, 杨瑞霞, 刘惜敏, 严胜骄, 林军, 有机化学, 2015, 35, 1754.) -
[18]
(a) Li, J.-Y.; Li, Q.-Y.; Jiang, B.; Tu, S.-J. RSC Adv. 2013, 3, 5056.
(b) Jiang, B.; Li, Y.; Tu, M.-S.; Wang, S.-L.; Tu, S.-J.; Li, G.-G. J. Org. Chem. 2012, 77, 7497.
(c) Kong, L.-B.; Yang, R.-X.; Du, X.-X.; Yan, S.-J.; Lin, J. Chin. J. Org. Chem. 2016, 36, 2437 (in Chinese). (孔令斌, 杨瑞霞, 杜璇璇, 严胜骄, 林军, 有机化学, 2016, 36, 2437.) -
[19]
(a) Makawana, J. A.; Patel, M. P.; Patel, R. G. Chin. Chem. Lett. 2012, 23, 427.
(b) Hundsdörfer, C.; Hemmerling, H. J.; Götz, C.; Totzke, F.; Bednarski, P.; Borgne, M. L.; Jose, J. Bioorg. Med. Chem. 2012, 20, 2282. -
[20]
Quiroga, J.; Trilleras, J.; Gálvez, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Cobo, J. Tetrahedron Lett. 2009, 50, 6404. doi: 10.1016/j.tetlet.2009.08.070
-
[1]
-
表 1 反应条件的优a
Table 1. Optimization of reaction conditions

Entry Solvent Catalyst T/℃ t/h Yieldb/% 1 EtOH — r.t. 4 N.R. 2 EtOH — Reflux 4 N.R. 3 EtOH Piperidine r.t. 2 63 4 EtOH Piperidine 40 1 86 5 EtOH Et3N 40 1 84 6 EtOH DBU 40 1 76 7 EtOH PPh3 40 4 N.R. 8 EtOH HOAc 40 1 N.R. 9 EtOH p-TSA 40 1 N.R. 10 EtOH L-Proline 40 1 72 11 Acetonitrile Piperidine 40 1 91 12 1, 4-Dioxane Piperidine 40 1 86 13 CH2Cl2 Piperidine 40 1 86 14 CH3OH Piperidine 40 1 78 15 H2O Piperidine 40 4 Trace a The reaction was performed with 3f (1.0 mmol), catalyst (0.3 mmol), and solvent (10 mL); b Isolated yield; N.R.=no reaction. 表 2 目标化合物4的合成
Table 2. Synthesis of target molecules 4

Entry R R1 3 4 Yieldb/% 1 4-Fluorophenyl H 3a 4a 87 2 3-Fluorophenyl H 3b 4b 89 3 4-Chlorophenyl H 3c 4c 86 4 4-Bromophenyl H 3d 4d 95 5 3-Bromophenyl H 3e 4e 92 6 Phenyl H 3f 4f 91 7 4-Methylphenyl H 3g 4g 87 8 3-Methylphenyl H 3h 4h 90 9 4-Methoxyphenyl H 3i 4i 91 10 4-Fluorophenyl CH3 3j 4j 93 11 4-Chloro phenyl CH3 3k 4k 91 12 3-Chlorophenyl CH3 3l 4l 84 13 2-Chlorophenyl CH3 3m 4m 90 14 4-Bromophenyl CH3 3n 4n 94 15 3-Bromophenyl CH3 3o 4o 90 16 Phenyl CH3 3p 4p 87 17 4-Methylphenyl CH3 3q 4q 89 18 3-Methylphenyl CH3 3r 4r 86 19 2-Methylphenyl CH3 3s 4s 82 20 4-Methoxyphenyl CH3 3t 4t 90 a The reaction was performed with 3 (1.0 mmol), catalyst (0.3 mmol), and solvent (10 mL); b Isolated yield. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 43
- 文章访问数: 3251
- HTML全文浏览量: 816




 下载:
下载:


 下载:
下载:

