 Figure 8.
TEM images of the triblock copolymer tBP1 and the size of micelles by DLS at 25 ℃ (a and d), 35 ℃ (b and e), and 45℃ (c and f) (cp=1 mg/mL)
Figure 8.
TEM images of the triblock copolymer tBP1 and the size of micelles by DLS at 25 ℃ (a and d), 35 ℃ (b and e), and 45℃ (c and f) (cp=1 mg/mL)

Micellization and Gelation of the Double Thermoresponsive ABC-type Triblock Copolymer Synthesized by RAFT
English
Micellization and Gelation of the Double Thermoresponsive ABC-type Triblock Copolymer Synthesized by RAFT
-
INTRODUCTION
During the past few decades, more and more attention is paid to stimuli responsive polymers, due to the widespread application and potential prospect[1-5]. And the stimuli responsive polymers would undergo some physicochemical changes such as their conformation, polarity, phase structure and composition, under external stimuli without the use of any additives. Temperature is the most common stimulus and various thermosensitive polymers have been widely investigated. Among these polymers, N-alkyl-substituted acrylamides polymers show thermally induced aggregation in water, in which the most well-known one is poly(N-isopropylacrylamide) (PNIPAM) with a lower critical solution temperature (LCST) at 32 ℃ [6-9]. Moreover, the polymers with oligo(ethylene glycol) side chains have been attracting a lot of attention as a new family of thermoresponsive polymers, especially poly(2-(2-methoxyethoxy) ethylmethacrylate) (PMEO2MA) and poly(oligo (ethylene glycol) methyl ether methacrylate)) (POEGMA)[10-12]. To the best of our knowledge, PNIPAM would collapse and aggregate above the LCST, meanwhile, it is stabilized by strong intramolecular and intermolecular ―NH···O=C hydrogen bonds as well as hydrophobic interactions among the entire polymer chains. But there is no strong hydrogen bond in POEGMA chains, and only weaker van der Waals interactions exist[13, 14]. The polymers with oligo(ethylene glycol) side chains display extremely sensitive and reversible phase separation[11]. Therefore, the copolymer P(MEO2MA-co-OEGMA) can be used as a promising PNIPAM alternative on account of good biological compatibility and widespread application[15].
Much attention is focused on the thermosensitive polymeric micelles owing to their enormous potential in biomedical application. Thereinto, double thermoresponsive block copolymers with two distinct phase transitions have been occasionally investigated[16-20]. For instance, Kotsuchibashi and co-workers obtained the copolymer of polyNIPAm-b-poly(NIPAAm-co-(N-(hydroxymethyl) acrylamide)), and the AB-type diblock copolymers showed doubly thermosensitive feature in aqueous solution at 35 and 47 ℃ [18]. In another example, block copolymers poly(N-isopropylacrylamide)-b-poly(N-vinylcaprolactam) (PNIPAM-b-PVCL) were studied by Hou et al[14]. Due to the collaborative and simultaneous dehydration of the blocks, only one LCST was observed for these block copolymers even if they had two thermoresponsive blocks[14]. In addition, a well-defined AB diblock copolymer, poly(2-(2-ethoxy) ethoxyethyl vinyl ether)-b-poly(2-methoxyethyl vinyl ether) (EOEOVE200-b-MOVE400) was synthesized, and its aqueous solution exhibited different viscoelastic stages upon heating. The phase separation was sensitive and reversible without hysteresis. But the diblock copolymers were synthesized by sequential living cationic copolymerization and the reaction conditions could not be easily controlled in the polymerization process[21].
Most of the multiple thermoresponsive block copolymers contain a poly(N-alkyl-substituted acrylamide) block, and they exist a significant hysteresis according to references[13, 14]. Among the thermoresponsive blocks, both PMEO2MA and POEGMA have turned out to be the most attractive polymers which present excellent reversible phase separation and biocompatibility. P(MEO2MA-co-OEGMA) is a random copolymer of MEO2MA and OEGMA, the LCST of which could be accurately shifted from 28 ℃ to 90 ℃ by increasing the molar fraction of OEGMA units in the copolymer chains[10, 22-24]. In addition, poly(ethylene glycol) (PEG) serves as a biomedical polymer material as a result of good water solubility and biocompatibility, approved by the US Food and Drug Administration (FDA)[25-30]. So in order to obtain the dual thermoresponsive polymers with extremely sensitive and reversible phase separation, employing the reversible addition-fragmentation chain transfer polymerization method (RAFT), one of the most successful controlled radical polymerization techniques, we synthesized a new double thermoresponsive ABC-type triblock copolymer that was named by PEG-b-PMEO2MA-b-P(MEO2MA-co-OEGMA). During the thermal-induced phase transitions, the copolymer which possesses two distinct LCSTs undergoes a two-step self-assembly due to the successive collapse of the thermosensitive blocks.
EXPERIMENTAL
Critical Micellization Concentration Determination
The critical micellization concentration (CMC) value is an important parameter for studying self-assembling behaviors. Critical micelle concentration was determined by fluorescence measurements at 35 and 45 ℃. Fluorescence spectra were recorded on a PE LS55 (America Perkin Elmer Corporation) luminescence spectrometer and pyrene was used as a hydrophobic fluorescent probe. Aliquots of pyrene solutions (10-3 mol/L in diethyl ether, 100 μL) were added into a series of volumetric flasks and they were placed a period of time to make the diethyl ether evaporate. 10 mL copolymer solutions with concentrations range from 0.001 mg/mL to 0.1 mg/mL were prepared by adding different volume copolymer solutions into the volumetric flasks containing the pyrene residue with the same concentration of 10-5 mol/L. The copolymer solutions were kept for 24 h to make the pyrene in the aqueous phase reach the solubilization equilibrium[6]. The emission spectra were recorded from 350 nm to 500 nm with an excitation wavelength of 331 nm; excitation and emission bandwidths were 2.5 and 10 nm, respectively; scanning speed was 300 nm/min[32].From the pyrene emission spectra, the intensities (peak height) of I1 (373 nm) and I3 (384 nm) were recorded, and the intensity ratio of I3/I1 was analyzed as a function of the polymer logarithmic concentration. Thus two CMC curves were obtained at 35 and 45 ℃. Corresponding to fluorescence measurement, the surface tension of the copolymer aqueous solutions was measured as a function of polymer concentrations at 35 and 45 ℃ using the automatic surface tension meter (DCAT 21, Germany Dataphysics Corporation). Plotting surface tension versus polymer concentration yields the CMC which indicated by intersection of the extrapolation.
Gel Permeation Chromatography
The molecular weight and molecular weight distribution (PDI) of the polymers were obtained by gel permeation chromatography (GPC) (Breeze, Waters, Milford, MA, USA). Tetrahydrofuran (THF) was employed as the mobile phase at 100 μL/min flow rate, and the system was calibrated by polystyrene standard. A series of polymer solutions in THF were prepared at a concentration of 4 mg/mL. The polymer solutions were filtered through a 0.45 μm oil phase pin type filter prior to measurement.
Transmittance Measurements of Copolymer Aqueous Solutions
The thermo-responsive behaviors of copolymer aqueous solutions were determined by measuring the optical transmittance on a UV-Vis spectrophotometer (TU-1901, Beijing Purkinje General Instrument Corporation, China) equipped with a peltier temperature control system. Copolymer aqueous solutions at a concentration range of 0.5-2 mg/mL were prepared by dissolving the polymers in double-distilled water. Light transmittance at different temperatures was continuously recorded at a wavelength of 500 nm, and heating rate of 1 K/min. The LCST was determined from 50% of the transmittance versus temperature plot.
Transmission Electron Microscopy Observation
The transmission electron microscopy (TEM) samples were prepared by dipping a carbon-coated copper grid (400 mesh) into the copolymer solutions preheated at different temperatures. After the samples dried in the corresponding temperatures, samples were stained with 1 wt% phosphattungstic acid aqueous solution, and also dried in the corresponding temperatures. The TEM analysis was then carried out on a JEOL JEM-2100 (Japan) instrument at an acceleration voltage of 200 kV to examine the particle characteristics.
Materials
Unless otherwise noted, all reagents were used without further purification. Monomethoxy poly(ethylene glycol) (mPEG, Mw=2000 g/mol) was purchased from TCI ((Shanghai) Development Co., Ltd.) and dried under a high vacuum before use. N-(3-(dimethylamino) propyl)-N-ethylcarbodiimide hydrochloride (EDC) and 4-(N, N-dimethylamino) pyridine (DMAP) were purchased from TCI ((Shanghai) Development Co., Ltd.). All solvents were distilled prior to use. CH2Cl2 was dried over CaH2, and 1, 4-dioxane was refluxed over sodium and distilled.
N, N-azobisisobutyronitrile (AIBN) was recrystallized from methanol. Monomers 2-(2-methoxyethoxy) ethyl methacrylate (MEO2MA, 95%, Mn=188 g/mol) and oligo (ethylene glycol) methyl ether methacrylate (OEGMA, 95%, Mn=475 g/mol) were also purchased from TCI ((Shanghai) Development Co., Ltd. Beijing Branch). All other reagents were purchased as reagent grade from commercial sources and used as received.
Synthesis of the mPEG-CTA macro-RAFT Agent
The synthesis of S-1-dodecyl-S'-(α, α'-dimethyl-α''-acetic acid) trithiocarbonate chain transfer agent was carried out according to the previously reported method in literatures[31, 32]. The yellow crystalline solid (trithiocarbonate chain transfer agent) was obtained in 72.8% yield. Macro-RAFT agent (mPEG-CTA) was synthesized by esterification of S-1-dodecyl-S'-(α, α'-dimethyl-α''-acetic acid) trithiocarbonate and monomethoxy poly(ethylene glycol). S-1-dodecyl-S'-(α, α'-dimethyl-α''-acetic acid) trithiocarbonate chain transfer agent (0.725 g, 2 mmol), mPEG (1.0 g, 0.5 mmol), and catalytic amount of DMAP (0.026 g, 0.225 mmol) were dissolved in 40 mL methylene dichloride. When the solution was homogenized by stirring, EDC solution in methylene dichloride (EDC 0.43 g, 2.25 mmol) was added in one portion into the solution. The esterification reaction proceeded under stirring at room temperature for 72 h. The by-product was washed twice in Na2CO3aqueous solution. After dried over anhydrous sodium sulfate for 24 h, the solution was concentrated and precipitated in an excess amount of diethyl ether[31]. Macro-RAFT agent (mPEG-CTA) with yellow color was obtained by filtration and dried under vacuum at room temperature for 24 h.
Sol-Gel Transition
The sol-gel transition temperatures were recorded by the test-tube inverting method (with a temperature increment of 2 K per step). Each sample of a given concentration was prepared by dissolving the copolymer in double-distilled water. The solution was gradually heated from 10 ℃ to 70 ℃ by intervals of 2 K. The 4 mL vials containing 2 mL of copolymer solutions were immersed in water bath at a designated temperature for 20 min. The sol (flow)-gel (no flow) phase transition temperatures of copolymer solutions were determined by the formation of a firm gel that remained intact when the tube was inverted by 180°.
Dynamic Light Scattering
Dynamic light scattering (DLS) was measured on a dynamic light scattering instrument (ZS90, Malvern, England) with a 4 mW He-Ne solid state laser (l=633 nm, detection angle: 90°). Dynamic light scattering was used to monitor the self-assembly behaviors of copolymer aqueous solutions at different temperatures. The laser wavelength was 633 nm, and the scattering angle was fixed at 90°. All copolymer solutions were prepared by filtration with a 0.45 μm millipore filter and the copolymer solutions were kept in a given temperature to reach the equilibrium prior to the measurements.
Synthesis of mPEG-b-PMEO2MA-b-P(MEO2MA-co-OEGMA)
Firstly macro-RAFT agent (mPEG-CTA) (0.0296 g, 0.0125 mmol), AIBN (0.0021 g, 0.0125 mmol)[33-35] and MEO2MA (0.94 g, 5 mmol) were dissolved in 1, 4-dioxane (7 mL) and bubbled with nitrogen gas for 2 h. The mixture was immersed in a preheated oil bath. The reaction temperature was set to 70 ℃, and the reaction was conducted for 60 min before terminating the reaction mixture by immersing it at ice bath. Next, a mixed solution containing MEO2MA (0.4799 g, 2.55 mmol), OEGMA (0.2138 g, 0.45 mmol) and 1, 4-dioxane (5 mL) was added into the reaction mixture in order to prepare the P(MEO2MA-co-OEGMA) block. This polymerization reaction was carried out at 70 ℃ for 10 h[36, 37]. The resulting copolymer was purified by dialysis against distilled water. Finally, the block copolymer was obtained by lyophilization.
1H-NMR Measurements
The 1H-NMR spectra of all copolymers were recorded on a Bruker 300 MHz spectrometer (300 MHz AVANCE, Bruker Corporation) using CDCl3 as the solvent. Chemical shifts (δ) relative to tetramethylsilane (TMS) were given.
Dynamic Rheological Analysis
The sol-gel transition of the copolymer aqueous solution was investigated by rheological experiments using a stress-controlled rheometer (TA Instruments Model AR-G2, UK). A parallel plate with a diameter of 20 mm was employed; the temperature was controlled by the bottom Peltier plate. In each measurement, 1 mL copolymer solution was loaded onto the plate. The data were collected under a controlled stress (1.0 × 10-5 N/cm2) and a frequency of 1.0 rad/s. The heating rate was 0.5 K/min and temperature ramp from 10 ℃ to 70 ℃.
RESULTS AND DISCUSSION
Transmission Electron Microscopic Measurement
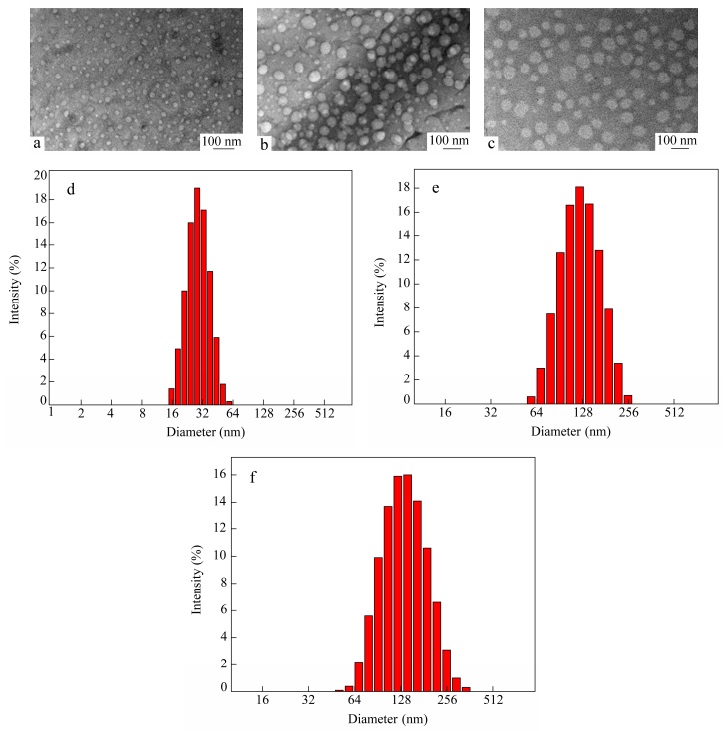
In order to visualize these micelles aggregation, the transmission electron microscopic measurements were carried out, and the obtained images are shown in Figs. 8(a), 8(b) and 8(c) at 25, 35 and 45 ℃, respectively. Meanwhile, Figs. 8(d), 8(e) and 8(f) display the particle size of the tBP1 aqueous solutions at the corresponding temperatures by DLS measurement. The white spots in Fig. 8(a) appear because the average particle size is about 30 nm, existing in coil for the single polymer chain (below the LCSTs)[42]. Figures 8(b) and 8(c) could be confirmed that the self-assembled micelles are well-dispersed as individual particles with a regularly spherical shape at 35 and 45 ℃. It demonstrates that the single polymer chains dissolved in aqueous solution undergo a collapse transition from expanded coil to compact globule with increasing temperature. Meanwhile, the diameter of micelle at 35 ℃ is relatively smaller than that of micelle at 45 ℃. Above the first LCST, the central PMEO2MA block dehydrates and forms the aggregated core surrounded by a shell of the peripheral mixed soluble PEG and P(MEO2MA-co-OEGMA) chains.
 Figure 8.
TEM images of the triblock copolymer tBP1 and the size of micelles by DLS at 25 ℃ (a and d), 35 ℃ (b and e), and 45℃ (c and f) (cp=1 mg/mL)
Figure 8.
TEM images of the triblock copolymer tBP1 and the size of micelles by DLS at 25 ℃ (a and d), 35 ℃ (b and e), and 45℃ (c and f) (cp=1 mg/mL)
While the temperature steps across the second LCST, the P(MEO2MA-co-OEGMA) block also undergoes dehydration, giving rise to the collapse from the micelles shell to the core, and only short PEG chains build the hydrophilic shell. Figures 8(d), 8(e) and 8(f) show that the particle size of the tBP1 are about 30, 120, 130 nm at 25, 35 and 45 ℃, respectively. It indicates that the results of DLS measurements are roughly consistent with that of TEM. But the particle sizes observed by TEM are slightly smaller than those measured by DLS measurement for the reason that the samples for TEM would collapse and shrink during the drying.
Dynamic Light Scattering Measurement
Dynamic light scattering (DLS) measurement was also performed to investigate the two-step self-organisation behavior by measuring the aggregate particle size of the ABC-type triblock copolymer aqueous solution. Different from the transmittance measurement, the particle size obtained by DLS includes not only the core but also the corona and its hydrated layer. Figure 7 shows that the particle size has a tendency to increase with increasing temperature. The triblock copolymer is fully hydrophilic and the average particle size is about 30 nm (T < 24 ℃). The value of particle size increases dramatically and reaches about 120 nm owing to the formation of micelles above the first LCST (T > 29 ℃). Then a plateau is recorded from 29 ℃ to 36 ℃, which suggests the stability of self-assemblies at the first stage. Because the dehydrated PMEO2MA core is stabilized by the hydrophilic mixed soluble PEG and P(MEO2MA-co-OEGMA) shells. The aggregate size decreases slightly within the temperature range and it indicates that when the temperature does not reach the second LCST, the interactions of contraction play a dominant role for triblock copolymer chains and the water in the loosely aggregated micelles is excluded out and slightly dense aggregated micelles are obtained[41]. Upon further heating to the second LCST, the change of particle size presents another plateau value. The change is small at the second stage and the value is about 130 nm, which is the result of a competition between the aggregation and contraction of triblock copolymer chains. It occurs that P(MEO2MA-co-OEGMA) segment transforms its hydrophilicity into hydrophobicity, leading to the particle size increases again (T > 39 ℃). Meanwhile, the particle size has a tendency of contraction as temperatures rise. Thus, the interactions of aggregation play an important role in triblock copolymer chains and the particle size is relatively larger at the second stage. Moreover, as for the copolymer solutions of different concentrations, the particle size also increases with the increasing concentration. The higher the concentrations of triblock copolymer solution there are, the stronger the hydrophobic interactions in intrachains and interchains are. In addition, the two temperatures which the self-assemblies occur at are slightly lower than those by transmittance measurements on account of that there is relatively low thermal energy dissipation in the process of heating. But it also supports effectively the two-stage assembly of the dual thermosensitive copolymer and demonstrates the change of particle size during the two-stage assembly.
Gelation Investigation of the ABC-type Triblock Copolymer
Critical Micellization Concentration and Critical Micellization Temperatures Determination
The critical micellar concentration (CMC) value is an important parameter for studying self-assembling behaviors of the triblock copolymer. The CMC of the triblock copolymer solution is verified by fluorescence probe technique using pyrene as a hydrophobic probe or the automatic surface tension meter. When the temperature and concentration are above the LCST and CMC, respectively, the triblock copolymer drives the formation of micelles consisting of a hydrophobic core surrounded by hydrophilic shell. Figure 9(a) uses the I3/I1 ratio of pyrene at 35 ℃ (above the first LCST) and 45 ℃ (above the second LCST) to assess the size of micelles at the values of CMC. Fluorescence intensity ratio (I3/I1) of pyrene varies with increasing copolymer concentration in the figure. Pyrene serves as a hydrophobic fluorescent probe, the copolymers adsorb a little pyrene below the CMC. While above the CMC, due to the formation of micelle, pyrene is transferred into a hydrophobic environment so that plenty of pyrene is trapped by the hydrophobic core, leading to increase of fluorescence intensity ratio (I3/I1). There is a wider hydrophobic domain, the concentration of pyrene in the micelles increases, and fluorescence intensity ratio (I3/I1) increases. Thus, critical micellar concentration is relatively low. As described by the TEM and DLS, the size of micelles at 45 ℃ is larger than that at 35 ℃. Thus the CMC would appear at lower concentration at 45 ℃ owing to the larger hydrophobic region. As shown in Fig. 9(a), the CMC at 45 ℃ (0.0035 mg/mL) is smaller than that at 35 ℃ (0.0052 mg/mL). In order to corroborate the CMC of the triblock copolymer at 35 and 45 ℃, we also use the automatic surface tension meter to measure the CMC. The surface tensions of the triblock copolymers aqueous solutions are measured as a function of polymer concentrations at 35 and 45 ℃. The critical micellization concentration is determined by intersection of the extrapolation of the two linear regimes in the figure of surface tensions versus polymer concentration. Amphiphilic triblock copolymer chains tend to aggregate to form micelles rather than migrate to the interface beyond the CMC. Therefore, the interfacial tension maintains almost constant above the CMC. If the size of micelles is large, the low concentration is needed when the triblock copolymer is full of the surface of aqueous solution to form the micelles. The CMC at 35 ℃ is larger than that at 45 ℃ which mainly corresponds to the CMCs measured by fluorescence probe technique. The CMCs measured by the automatic surface tension meter are shown in Fig. 9(b). The results indicate that the size of micelles is different during the two thermal-induced phase transition.
In addition, the fluorescent spectra of pyrene are recorded for tBP1 triblock copolymer solution at different temperatures. Pyrene is used as a hydrophobic probe to evaluate the polarity of the microenvironment surrounding the pyrene molecules. As mentioned above, pyrene is inclined to transfer into the hydrophobic domain if it is present in the aqueous solution. Once pyrene enters these domains, there will be a red shift in the emission spectrum. The I3/I1ratio of pyrene appears two platforms in the temperature range from 15 ℃ to 45 ℃ in Fig. 10. In detail, the fluorescent spectra of pyrene have two main transitions: first, the value rose from 0.73 to 0.77, followed by the second increase from 0.77 to 0.79, the corresponding temperature ranges of the two transitions are 24-29 ℃, 36-41 ℃, respectively. The two temperature ranges are in conformity with that by DLS measurement. The result demonstrates the systematic formation of a hydrophobic zone and the two critical micellization temperatures illustrates the two stable self-assembly in the triblock copolymer solution with the increasing temperature.
Synthesis and Characterization of Polymers
The ABC-type double thermoresponsive triblock copolymer PEG-b-PMEO2MA-b-P(MEO2MA-co-OEGMA) was synthesized by RAFT. Overall synthesis experiments are illustrated in Scheme 1. The chemical structures of the CTA, macro-RAFT agent mPEG-CTA, triblock copolymer PEG-b-PMEO2MA-b-P(OEGMA-co-MEO2MA) were proven by 1H-NMR and FTIR spectra. Figure 1 shows the FTIR spectra of the CTA, PEG, macro-RAFT agent PEG-CTA. In the FTIR spectrum of the CTA (Fig. 1a), the peaks at 3423 and 1730 cm-1 are attributed to the hydroxyl group and the C=O stretching vibration, and the peak at 1065 cm-1 is the absorption of the C=S vibration in the trithiocarbonate units. The FTIR spectrum of the PEG is shown in Fig. 1(b) and the peak at 3436 cm-1 is the signal of the hydroxyl group stretching vibration, and the peaks at 2886 and 1466 cm-1 are attributed to C―H bonds stretching vibration and flexure vibration, respectively. Compared with Figs. 1(a) and 1(b), the peak of hydroxyl group disappears, and there is a C=O stretching vibration emerged at 1731 cm-1 in the Fig. 1(c)[31]. Because the esterification reaction proceeds between the carboxyl of CTA and the hydroxyl of the PEG, thus C=O stretching vibration exists in the FTIR spectra of macro-RAFT agent PEG-CTA. In the Fig. 2(B), different from the 1H-NMR spectrum of CTA (Fig. 2A), some new peaks are apparent in that of the macro-RAFT agent: the peak at δ=4.18 (peak“b”), corresponds to the methylene proton of―CH2―O―(C=O), and the signal at δ=3.58 (peak“c”) is assigned to the proton in the methylene of the PEG chain[38, 39]. As to the double thermoresponsive triblock copolymer PEG-b-PMEO2MA-b-P(OEGMA-co-MEO2MA), the peaks at δ=0.8-1.2 (a) and 1.8-1.9 (b) are attributed to the proton signals of methyl and methylene groups in the main chain, respectively. The resonance peaks at δ=3.40, 3.66, and 4.12 (peak“c” “d” “e”), are assigned to methyl protons of―OCH3, methylene protons of―OCH2CH2O―, and―CH2OOC―in the pendant oxyethylene side chains of copolymer (Fig. 2C). Therefore, the double thermosensitive triblock copolymer was successfully synthesized due to the emerging relevant peaks in the figures of 1H-NMR and FTIR spectra. The molecular weight and molecular weight distribution of the polymers were determined by GPC. Characteristics of all the samples are summarized in Table 1.
Copolymers The degree of polymerization [M]0:[O]0a Mnb Mwb PDI b LCSTs c (℃) dBP1 E45bM400 - 67100 102300 1.525 28 dBP2 E45b(MO)240 85:15 32700 39200 1.198 44 tBP1 E45bM400b(MO)240 85:15 99900 137500 1.377 29/39 tBP2 E45bM400b(MO)240 80:20 97200 130400 1.342 29/48 tBP3 E45bM400b(MO)240 90:10 94300 120300 1.276 37 tBP4 E45bM200b(MO)240 85:15 65600 88000 1.334 40 tBP5 E45bM400b(MO)120 85:15 82600 99200 1.20 31 dBP1 and dBP2 represent the PEG-b-PMEO2MA and PEG-b-P(MEO2MA-co-OEGMA). tBP represents the PEG-b-PMEO2MA-b-P(MEO2MA-co-OEGMA).
a[M]0:[O]0 is the initial molar ratio of MEO2MA and OEGMA; bDetermined by GPC in THF; cDetermined by UV transmittance measurementTable 1. Polymerization conditions and properties for the polymersMicellization Investigation of the Double Thermoresponsive ABC-type Triblock Copolymer
UV transmittance measurement
Prior to researching the double thermoresponsive triblock copolymer, we confirmed the thermoresponsive behaviors of PEG-b-PMEO2MA (dBP1), PEG-b-P(MEO2MA-co-OEGMA) (dBP2) and then we mainly investigated double thermoresponsive triblock copolymer PEG-b-PMEO2MA-b-P(MEO2MA-co-OEGMA) (tBP). As shown in Fig. 3, the thermoresponsive behaviors of dBP1, dBP2 and tBP1 in aqueous solutions were monitored by transmittance measurements.The LCSTs of dBP1 and dBP2 are 28 and 44 ℃, respectively, corresponding to the previous report in the literature[10]. Compared with them, the ABC-type triblock copolymer tBP1 exhibits two cloud points at around 29 and 39 ℃ during the heating process, mainly in accordance with the LCSTs of the thermosensitive blocks. The first cloud point at around 29 ℃ is based on the PMEO2MA segment and slightly higher than the cloud point of dBP1, indicating that the PMEO2MA segment increases in hydrophilicity when it is coupled with P(OEGMA-co-MEO2MA) segment. By the contrast and analysis of the three polymers, because both PEG and P(OEGMA-co-MEO2MA) are hydrophilic blocks (T < 39 ℃), and there are more hydrogen bonds between ether oxygen groups on P(OEGMA-co-MEO2MA) side chains and water molecules in aqueous solution. Destroying the solubilization equilibrium between hydrophilic polymer-water interactions and hydrophobic polymer-polymer interactions needs more energy, hence the first LCST is higher. On the other hand, the second cloud point at 39 ℃ of tBP1 is lower than that of dBP2 (44 ℃) which has the same molar fraction of OEGMA. Because the cloud point of P(OEGMA-co-MEO2MA) segment might be influenced by the hydrophobicity of PEG-b-PMEO2MA segment so that it shifts to lower temperature[18]. The outcome illustrates that the LCSTs are influenced by the relative hydrophilicity of the thermosensitive blocks.
Figure 4 shows the transmittance curves for triblock copolymer aqueous solutions and tBP1, tBP2 and tBP3 contain different molar fractions of MEO2MA and OEGMA (Table 1). It indicates that the feed ratios of MEO2MA and OEGMA monomers play an important role in the second LCST. There are two LCSTs for the tBP1 (29 and 39 ℃) and tBP2 (29 and 48 ℃), but tBP3 only has one LCST (37 ℃). Because OEGMA has an average of nine ethylene glycol repeating units, the hydrogen bonds interactions between polymers and surrounding water molecules are more and stronger than MEO2MA. Thus, the higher the molar fraction of OEGMA in the P(OEGMA-co-MEO2MA) segment there is, the higher the second LCST is. As a result, the values of first LCST and second LCST have no great difference for tBP3 with the smaller molar fraction of OEGMA units in the P(OEGMA-co-MEO2MA) segment, leading to the collaborative and simultaneous dehydration.
Figure 5 compared the thermo-responsive behaviors of tBP1, tBP4 and tBP5 in aqueous solutions and these copolymers have different degrees of polymerization (Table 1). As seen in Fig. 5, only tBP1 endows the double thermosensitivity while tBP4 and tBP5 don’t show this property. Due to the simultaneous dehydration and aggregation of the two thermosensitive blocks, a single LCST for tBP4 and tBP5 appears in the transmittance curve. The value of LCST is close to that of block with the higher degree of polymerization, because the hydrophobic interactions of the block play a crucial role for the LCST. It demonstrates that the different degrees of polymerization have an impact on the double thermosensitivity of triblock copolymers.
In Fig. 6, the transmittance of tBP1 at a series of different concentrations aqueous solutions are measured. The ABC-type triblock copolymer solutions show the clear decreasing of transmittance at around 29 and 39 ℃, and the two LCSTs almost keep constant. Some literatures have been reported that solution concentration has an effect on the LCST of the polymer aqueous solution: increasing the solution concentration, the LCST value would decrease and shift the cloud point toward lower temperature. Therefore, in order to measure the thermoresponsive behaviors precisely, it is necessary to have a suitable concentration that provides a sufficient amount of micelle aggregate particles so that there is no kinetically restricted aggregation during the thermally induced phase transition[40]. Two clearly sharp phase transitions at 29 and 39 ℃ are observed at low concentration (0.5-2 mg/mL). It is beneficial for the research of micelles in the range of these concentrations. Through the discussion, the LCSTs of tBP1 are favorable for biomaterials in a broad range of potential applications. Due to the research results, the dual thermosensitive of triblock copolymer tBP1 is selected for the further study.
Thermo-induced sol-gel transition of the triblock copolymer
Test tube inverting method is used to measure the sol-gel-syneresis phase transition of ABC-type triblock copolymer. Generally speaking, the sol (flow) and gel (non flow) are differentiated by the flow characteristics when the vial containing solution is inverted at a given temperature. Figure 11 gives the sol-gel-syneresis phase transition diagram that the sol-gel transition temperature is plotted against the triblock copolymer concentration. The critical gelation concentration (CGC) is defined as the minimum concentration in aqueous solution at which the gelation behavior could be observed. In the case of micellar packing, the minimum gelation concentration depends on micelle aggregation number. A three-dimensional micellar gel of the 10 wt% aqueous solution is obtained with increasing temperature. Photographs of the state for the tBP1 aqueous solutions (15 wt%) at different temperatures are shown in Fig. 11 (below). When the temperature rises linearly from 10 ℃ to 60 ℃, the self-assembly system with double thermosensitive triblock copolymer emerges four different states: the transparent sol, turbid sol, turbid gel and the syneresis. Triblock copolymer is fully soluble transparent sol (T < the first LCST). When the temperature reaches to the first LCST, the hydrogen bonds between the PMEO2MA segment and water molecules are destroyed so that the triblock copolymer appears as turbid sol, indicating the micelles form. Continued to increase the temperature to the second LCST, P(MEO2MA-co-OEGMA) segment also becomes hydrophobic and collapses, a gel state is observed. For a micellar gel, the gelation of copolymer solution is simply due to micelle close-packing and occurs at the point where micellar shell (PEG) starts to overlap and self-associate, or by intermicellar bridging. However, the syneresis would arise as the temperature continues to increase. In addition, concentration of triblock copolymer has an effect on the sol-gel transition temperature, and the temperature would become lower with the increasing of concentration. Because when the concentration increases, the number of micelles forming above its LCST would be larger, and so it is the probability of hydrophobic segment aggregation, thus the temperature of sol-gel transition would become lower. It is important to note that these thermo-induced sol-gel transitions are reversible.
Rheological studies
Dynamic rheological measurement also confirms the sol-gel transition of the triblock copolymer by giving the storage modulus G' and loss modulus G". We carried out an oscillatory shear experiment at a fixed frequency of 1 Hz in a heating ramp at a heating rate of 0.5 K/min. The G' and G" of the triblock copolymer solutions are studied as a function of the temperature. The abrupt increase of the storage modulus G' indicates the occurrence of sol-gel transition (Fig. 12a). The G' also increases as the concentrations of the triblock copolymer solutions increase from 10 wt% to 20 wt%. Meanwhile, the sol-gel transition temperature reduces from 38 ℃ to 29 ℃. It is explained that the sol-to-gel transition temperature varies depending on the concentrations. With the increasing concentrations, the interactions between intrachain and interchain of the triblock copolymer increase so that the solutions have an enhanced hydrophobicity and the sol-gel transition temperature decreases. Figure 12(b) shows that the changes of G' and G" for the triblock copolymer are recorded as the temperatures arise. Below the two LCSTs, the values of G' and G" are small, even G" is larger than G' indicating that the sample is a viscous liquid. Both G' and G" increase and G' becomes slightly greater than G" with the rising temperature, which indicates that the viscous liquid turns into a gel. The crossover, G'=G", is usually regarded as a sign of the sol-gel transition. But in the Fig. 12(b), there are two crossovers at 28 and 36 ℃. The values of G' and G" are pretty much the same in the range of the temperatures. Due to the fact that the ABC-type triblock copolymer is a double thermosensitive copolymer, the PMEO2MA block begins to dehydrate and form micelles at the first LCST. Further heated to the second LCST, the P(MEO2MA-co-OEGMA) block dehydrates and the PEG chains self-associate to form hydrogel. As the temperature continues to increase, the values of G' and G" have a lower tendency. It indicates that the syneresis occurs for the hydrogel.
With respect to the essential reason of the thermally induced phase transition of the double thermoresponsive triblock copolymer in aqueous solution, combining transmittance analysis, DLS, TEM observation of morphology changes and vial inversion tests, a mechanism about the thermally induced micellization and sol-gel transitions for tBP1 aqueous solution is proposed and depicted in Fig. 13.
CONCLUSIONS
A novel ABC-type triblock copolymer PEG-b-PMEO2MA-b-P(MEO2MA-co-OEGMA) was synthesized successfully using reversible addition-fragmentation chain transfer (RAFT) polymerization. The triblock copolymer containing PMEO2MA and P(MEO2MA-co-OEGMA) showed a two-step self-organisation behavior at the two LCSTs (29 and 39 ℃) due to the stepwise aggregation of thermoresponsive blocks. Depending on temperature and concentration changes, the triblock copolymer was either soluble, micelles, or gelation. The driving force to form the micelles is owing to the hydrophobic interactions of the thermosensitive segment, and the gelation is due to micelles close-packing or micellar shell (PEG) start to overlap and self-associate by intermicellar bridging. The combination of the different techniques helps to provide a better understanding for the stepwise aggregation process, which is more complex than those of the single thermosensitive polymers. In addition, PEG served as a biomedical polymer material with good water solubility and biocompatibility, the polymers of MEO2MA and OEGMA with oxyethylene units exhibit extremely sensitive and reversible phase separation. Based on these results, the dual thermosensitive triblock copolymer might be the biomaterials in a broad range of potential applications.
-
-
[1]
Joo, J.H., Ko, D.Y., Moon, H.J., Shinde, U.P., Park, M.H. and Jeong, B., Biomacromolecules, 2014, 15:3664 doi: 10.1021/bm500942p
-
[2]
Shi, S., Yu, Y., Wang, T., Wang, Q.M., Wang, C. and Kuroda, S., Chinese J. Polym. Sci., 2014, 32(5):524 doi: 10.1007/s10118-014-1435-8
-
[3]
Wu, Q.J.Y, Wang, R., Zhou, Y., Huang, Y.Q., Ghosh, R. and Chen, X.N., Chinese J. Polym. Sci., 2015, 33(7):1048 doi: 10.1007/s10118-015-1655-6
-
[4]
Wang, X.L., Ma, X.Y. and Zang, D.Y., Soft Matter, 2013, 9:443 doi: 10.1039/C2SM26797G
-
[5]
Iatridi, Z., Mattheolabakis, G., Avgoustakisb, K. and Tsitsilianis, C., Soft Matter, 2011, 7:11160 doi: 10.1039/c1sm06185b
-
[6]
Xiao, J.J., Li, X.B., Wang, X., Yi, C.W. and Su, S.P., Chinese J. Polym. Sci., 2015, 33(3):456 doi: 10.1007/s10118-015-1598-y
-
[7]
Tang, Y.F., Zhang, S.M., Wang, M., Zhu, J.L., Sun, T.M. and Jiang, G.Q., J. Polym. Res., 2014, 21:390 doi: 10.1007/s10965-014-0390-y
-
[8]
Yang, L.L., Zhang, J.M., He, J.S., Zhang, J. and Gan, Z.H., Chinese J. Polym. Sci., 2015, 33(12):1640 doi: 10.1007/s10118-015-1703-2
-
[9]
Cheng, C., Wei, H., Shi, B.X., Cheng, H., Li, C., Gu, Z.W., Cheng, S.X., Zhang, X.Z. and Zhuo, R.X., Biomaterials, 2008, 29:497 doi: 10.1016/j.biomaterials.2007.10.004
-
[10]
Lutz, J.F., J. Polym. Sci., Part A:Polym. Chem., 2008, 46:3459 doi: 10.1002/(ISSN)1099-0518
-
[11]
Trzebicka, B., Szweda, D., Rangelov, S., Kowalczuk, A., Mendrek, B., Wesołek, A.U. and Dworak, A., J. Polym. Sci., Part A:Polym. Chem., 2013, 51:614 doi: 10.1002/pola.26410
-
[12]
Lutz, J.F. and Hoth, A., Macromolecules, 2006, 39:893 doi: 10.1021/ma0517042
-
[13]
Sun, S.T. and Wu, P.Y., Macromolecules, 2013, 46:236 doi: 10.1021/ma3022376
-
[14]
Hou, L. and Wu, P.Y., Soft Matter, 2014, 10:3578 doi: 10.1039/c4sm00282b
-
[15]
Lutz, J.F., Weichenhan, K., Akdemir, Ö. and Hoth, A., Macromolecules, 2007, 40:2503 doi: 10.1021/ma062925q
-
[16]
Boyer, C., Whittaker, M.R., Luzon, M. and Davis, T.P., Macromolecules, 2009, 42:6917 doi: 10.1021/ma9013127
-
[17]
Weiss, J., Böttcher, C. and Laschewsky, A., Soft Matter, 2011, 7:483 doi: 10.1039/C0SM00531B
-
[18]
Kotsuchibashi, Y., Yamamoto, K. and Aoyagi, T., J. Polym Sci., Part A:Polym. Chem., 2008, 46:6142 doi: 10.1002/pola.v46:18
-
[19]
Jochum, F.D., Roth, P.J., Kessler, D. and Theato, P., Biomacromolecules, 2010, 11:2432 doi: 10.1021/bm1006085
-
[20]
Savoji, M.T., Strandman, S. and Zhu, X.X., Macromolecules, 2012, 45:2001 doi: 10.1021/ma2027269
-
[21]
Sugihara, S., Kanaoka, S. and Aoshima, S., Macromolecules, 2005, 38:1919 doi: 10.1021/ma048409m
-
[22]
Synytska, A., Svetushkina, E., Puretskiy, N., Stoychev, G., Berger, S., Ionov L., Bellmann, C., Eichhorn, K.J. and Stamm, M., Soft Matter, 2010, 6:5907 doi: 10.1039/c0sm00414f
-
[23]
Jochum, F.D., Borg, L., Roth, P.J. and Theato, P., Macromolecules, 2009, 42:7854 doi: 10.1021/ma901295f
-
[24]
Li, N., Qi, L., Shen, Y., Li, Y.P. and Chen, Y., Appl. Mater. Interfaces, 2013, 5:12441 doi: 10.1021/am403510g
-
[25]
Chang, L.L., Liu, J.J., Zhang, J.H., Deng, L.D. and Dong, A.J., Polym. Chem., 2013, 4:1430 doi: 10.1039/C2PY20686B
-
[26]
Han, D.K. and Hubbell, J.A., Macromolecules, 1997, 30:6077 doi: 10.1021/ma970302u
-
[27]
Yue, G.L., Cui, Q.L., Zhang, Y.X., Wang, E.J. and Wu, F.P., Chinese J. Polym. Sci., 2012, 30(5):770 doi: 10.1007/s10118-012-1179-2
-
[28]
Chen, J., Spear, S.K., Huddleston, J.G. and Rogers, R.D., Green Chem., 2005, 7:64 doi: 10.1039/b413546f
-
[29]
Wang, L.H., Wang, J., Gao, X.Y., Liang, Z.Y., Zhu, B.K., Zhu, L.P. and Xu, Y.Y., Polym. Chem., 2014, 5:2836 doi: 10.1039/c3py01619f
-
[30]
Seidia, F. and Heshmati, P., Chinese J. Polym. Sci., 2015, 33(1):192 doi: 10.1007/s10118-015-1561-y
-
[31]
Chen, J.C., Liu, M.Z., Chen, C., Gong, H.H. and Gao, C.M., Appl. Mater. Interfaces, 2011, 3:3215 doi: 10.1021/am2007189
-
[32]
Lai, J.T., Filla, D. and Shea, R., Macromolecules, 2002, 35:6754 doi: 10.1021/ma020362m
-
[33]
Puttick, S., Irvine, D.J., Licence, P. and Thurecht, K.J., J. Mater. Chem., 2009, 19:2679 doi: 10.1039/b817181p
-
[34]
Wang, L.P., Wang, Y.P., Acta Chimica Sinica, 2007, 65:737
-
[35]
Gody, G., Rossner, C., Moraes, J., Vana, P., Maschmeyer, T. and Perrier, S., J. Am. Chem. Soc., 2012, 134:12596 doi: 10.1021/ja3030643
-
[36]
Liu, T. and Liu, S.Y., Anal. Chem., 2011, 83:2775 doi: 10.1021/ac200095f
-
[37]
Dai, X.H., Hong, C.Y. and Pan, C.Y., Macromol. Chem. Phys., 2012, 213:2192 doi: 10.1002/macp.v213.20
-
[38]
Chen, J.C., Liu, M.Z., Gao, C.M., Lu, S.Y., Zhang, X.Y. and Liu, Z., RSC Adv., 2013, 3:15085 doi: 10.1039/c3ra41832d
-
[39]
Hwang, M.J., Suh, J.M., Bae, Y.H., Kim, S.W. and Jeong, B., Biomacromolecules, 2005, 6:885 doi: 10.1021/bm049347a
-
[40]
Cui, Q.L., Wu, F.P. and Wang, E.J., J. Phys. Chem. B, 2011, 115:5913 doi: 10.1021/jp200659u
-
[41]
Cao, Y., Zhao, N., Wu, K. and Zhu, X.X., Langmuir, 2009, 25:1699 doi: 10.1021/la802971s
-
[42]
Peng, B.L., Grishkewich, N., Yao, Z.L., Han, X., Liu, H.L. and Tam, K.C., ACS Macro. Lett., 2012, 1:632 doi: 10.1021/mz300135x
-
[1]
-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 0
- 文章访问数: 915
- HTML全文浏览量: 45














 下载:
下载:

