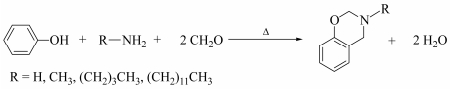 Figure Scheme 1.
Preparation of benzoxazine monomers
Figure Scheme 1.
Preparation of benzoxazine monomers

Surface Properties and Hydrogen Bonds of Mono-functional Polybenzoxazines with Different N-substituents
English
Surface Properties and Hydrogen Bonds of Mono-functional Polybenzoxazines with Different N-substituents
-
Key words:
- Polybenzoxazine
- / Mono-functional
- / N-substituents
- / Surface free energy
- / Hydrogen bonding
-
INTRODUCTION
Polymer materials with low surface free energy played an important role in numerous fields, including aviation, super hydrophobic surfaces, bio-coatings, environmental protection and pollution prevention[1-7]. Polybenzoxazines were a fast developing series of low-surface-free-energy materials[8-10] possessed many fascinating advantages such as minimal moisture absorption[11-13], good thermal stability[14-16], high char yield[17-19], and molecular design flexibility[17, 20-27]. These excellent performances had attracted great attention[28-35].
The polymerization of benzoxazines was performed by thermal open-ring activation, which took place through thermally activated ring opening of the cyclic benzoxazine structure. During the polymerization, intra-and intermolecular hydrogen bonding formed and transformed continuously[36-38], resulting in the changes of the polybenzoxazine films’surface free energy[39, 40]. Wang et al. reported that strong intramolecular hydrogen bonding between the hydroxyl groups of polybenzoxazines decreased their surface free energies, whereas which could be increased by the intermolecular hydrogen bonding[39]. After proposal of this theory, many researchers contributed to the low surface free energy films through reducing proportion of intermolecular hydrogen bonding interactions[41-44]. Apart from this, the alkyl group of primary amine also had potential contribution to the surface free energy[40, 45]. Ishida et al. found that the amine groups could affect the hydrogen-bonding structures of the model benzoxazine dimers[36, 45], while the compactness of a network structure was related to both the basicity and bulkiness of the functional amines[46]. Dong et al. systematically investigated the effect of N-substituents on the surface free energy and hydrogen bonding network structure of di-functional polybenzoxazine[40]. Their results showed that both the alkyl group and the proportion of intermolecular hydrogen bonding had an effect on the surface energy of the polybenzoxazines, and a transformation mechanism of the intermolecular and intramolecular hydrogen bonding during the progress of cure was proposed simultaneously.
Most studies about the hydrogen bonding network were based on the di-functional benzoxazine. However, the polymerization of mono-functional benzoxazines was different from that of di-functional ones. Therefore, their hydrogen bonding network structure would be different[47-50]. Mono-functional benzoxazine had better fluidity property, which was more conducive to the film forming, so the study of monofunctional benzoxazine was important to guide the practical application. In this study, a series of mono-functional benzoxazine monomers with different N-substituted groups were synthesized by phenol, formaldehyde, and various amines (ammonia, methylamine, n-butylamine, dodecylamine), named 3, 4-dihydro-3-H-2H-1, 3-benzoxazine (P-am), 3, 4-dihydro-3-methyl-2H-1, 3-benzoxazine (P-m), 3, 4-dihydro-3-n-butyl-2H-1, 3-benzoxazine (P-b), and 3, 4-dihydro-3-dodecyl-2H-1, 3-benzoxazine (P-da) respectively. The N-substituents effects and hydrogen bonding network of these mono-functional polybenzoxazine were studied by contact angle measurement and FTIR curve fitting, and the results demonstrated that both the N-substituent alkyl group and the fraction of intermolecular hydrogen bonding had an effect on the surface free energy.
EXPERIMENTAL
Thermogravimetric Analysis
The thermal stability was tested by an SDT Q600 V8.3 Build 101 thermalgravimetric analyzer. The polybenzoxazine samples were heated from room temperature to 800℃ at a heating rate of 10 K·min-1 under N2 atmosphere, and the thermal degradation temperature was obtained when the weight loss reached 5 wt%.
Contact Angle Analysis
The static contact angles of these films were measured by an OCA20 optical goniometer from Dataphysics. The testing liquids were deionized water, ethylene glycol, and diiodomethane (99%), and 2μL droplet was used with ellipse fitting for CA test. 5 droplets were measured at different regions for each sample, and the static contact angle data were the average values.
Surface free energy was deduced from the contact angle values, and the calculation was based on the OWRK mode in the Owens’s three-liquid method[51].
Nuclear Magnetic Resonance Spectroscopy
The NMR spectra of all the benzoxazine monomers were recorded on a Varian Mercury Plus 400 MHz NMR spectrometer in deuterated chloroform with tetramethylsilane (TMS) as the external reference.
Synthesis of Benzoxazine Monomer
The synthesis method of benzoxazine monomers is shown in Scheme 1[47]. 0.4 mol formaldehyde dissolved in 80 mL dioxane, was added in an four-necked flask around ice-water bath. Then 0.2 mol amine (13.62 g 25% ammonia, 24.88 g 25% aqueous solution of methylamine, 14.62 g of n-butylamine, and 37.07 g of dodecylamine) dissolved in 20 mL dioxane was slowly dosed, meanwhile the temperature was maintained at 10℃ or less. After stirring 10 min, 0.2 mol phenol (19 g) dissolved in 100 mL dioxane was added, and refluxed for 6 h. After cooling to room temperature, the mixture was dissolved in ether. Then, it was washed with sodium hydroxide solution for three times, followed by wash with water, then dried with Na2SO4. After evaporation of the solvents, the benzoxazine monomers were obtained as transparent, viscous, and yellowish liquids. The yields of benzoxazine monomers P-am, P-m, P-b and P-da were 27.74%, 30.97%, 57.26% and 30.58%, respectively.
Preparation of Benzoxazine Monomer Films and Polybenzoxazine Films
The P-am, P-m, P-b and P-da monomers were dissolved into tetrahydrofuran to get the 10 wt% solutions, and then filtered by 0.2μm microfiltration membranes. Then these solutions were spin-coated onto the treated glass substrates. The treatment method of the glass substrates (1 cm×1 cm) was as follows: soaked in the cleanser solution overnight, ultrasonic washed by water, ethanol, acetone for 15 min separately, and then dried with the nitrogen gas. Then the filtered solutions were spin-coated onto the treated glass substrates at a speed of 1500 r/m for 45 s, and dried in the vacuum oven at 60℃ for 1 h. Then the monomer films were prepared. After curing for another 1 h at 200℃, the polybenzoxazine films were obtained. These polymers were named as poly(P-am), poly(P-m), poly(P-b) and poly(P-da).
Materials and Chemical Reagents
Phenol, formaldehyde (37% in water), methylamine, n-butylamine, dodecylamine and tetrahydrofuran were purchased from Shanghai Lingfeng Chemical Reagent. Ammonia was purchased from Hubei Debang Chemical Reagent. All chemicals were of analytically pure grade.
Fourier Transform Infrared Spectroscopy
The transformation of the chemical interactions during the curing process could be demonstrated by the FTIR of the benzoxazine films cured at different temperatures for different time. The FTIR spectra were recorded by a Nicolet iS10 spectrometer from Thermo Fisher Scientific with 32 scans at 1 cm-1 resolution. The samples of benzoxazine monomers were prepared by dropping P-am, P-m, P-b and P-da onto KBr tablets, and the samples of the polybenzoxazine films were cured at 200℃ for another 1 h.
Differential Scanning Calorimetry
The thermal curing curves of the four benzoxazine monomers were tested by a Pyris Diamond DSC. 3-5 mg benzoxazine monomer samples were heated from 50℃ to 250℃ at a heating rate of 10 K·min-1 under N2 atmosphere. The heat flow curves show the thermal curing process of the benzoxazine monomers.
RESULTS AND DISCUSSION
Thermal Stability of Poly(P-am), Poly(P-m), Poly(P-b) and Poly(P-da)
The polybenzoxazines’thermal stabilities were characterized by TGA. Poly(P-am), poly(P-m), poly(P-b), and poly(P-da) were cured at 200℃ for 1 h, then heated under N2 atmosphere from ambient temperature to 800℃ at the heating rate of 10K·min-1. The results are shown in Fig. 8 and Table 5.
Resin sample Temperature at 5 wt% loss (℃) Temperature at 10 wt% loss (℃) Char yield at 800℃(%) P-am 229 288 49.60 P-m 250 298 51.90 P-b 235 255 33.44 P-da 236 256 18.15 Table 5. Thermal resistance of polybenzoxazine measured by TGAAs shown in Fig. 8, a three-stage weight loss process was observed in the TGA result of poly(P-am), while there was a two-stage weight loss process of poly(P-m), poly(P-b), and poly(P-da). The electron donating effect of alkyl chain could strengthen the integration of N atoms and substituents. Meanwhile, the first step of degradation was due to Mannich base cleavage (the cleavage of the C―N bond), resulting in the release of Schiff base (C=N) as reported in the literature[40]. The Schiff base in the P-am polybenzoxazine was H2C=NH with no alkyl chain linked with N atom, so that it exhibited a TGA curve different from others.
As shown in Table 5, the temperature at 5 wt% loss of P-m, P-b and P-da polybenzoxazines decreased with increasing the length of N-substituent alkyl chain. That was because the strong electron donating effect of alkyl chain could make Mannich base cleavage more easily. The char yields of these polybenzoxazines are also listed in Table 5, revealing that P-am and P-m polybenzoxazines have good thermal stability.
Synthesis and Characterization of Benzoxazine Monomers
Benzoxazine monomers were prepared from conventional raw materials through Mannich condensation reaction.
The chemical structures of these benzoxazine monomers were confirmed by FTIR and 1H-NMR. The FTIR spectra of P-am, P-m, P-b and P-da benzoxazine monomers are shown in Fig. 1 respectively.
As shown in Fig. 1, the FTIR spectra of these benzoxazine compounds demonstrated that the characteristic absorption bands of P-am, P-m, P-b and P-da appeared at 960, 930, 929, and 931 cm-1, respectively. The C―O―C asymmetric stretching bands were observed at 1230, 1253, 1219 and 1255 cm-1, respectively, and the symmetric stretching bands were observed at 1026, 1020, 1033 and 1034 cm-1, respectively. The CH2 wagging bands were observed at 1360, 1330, 1331 and 1376 cm-1, respectively.
The thermal curing curves of the four benzoxazine monomers are shown in the Fig. 2. The results indicated that the curing temperature decreased with the increase of the N-substituent length, and the curing temperatures of P-am, P-m, P-b and P-da were 229.42, 221.76, 213.96 and 192.62℃, respectively.
The chemical structures of the benzoxazine monomers were also confirmed by 1H-NMR, and the results are summarized in Table 1.

H P-am P-m P-b P-da a-d K6.75-7.25T K6.70-7.20T K6.77-7.11T K6.75-7.12T e 4.10 3.96 3.98 3.98 f 4.89 4.87 4.85 4.86 
g 1.23 1.21 2.74 2.73 1.54 1.55 1.34 1.27 0.91 0.87 Table 1. 1H-NMR spectra of P-am, P-m, P-b and P-daSurface Properties of the Polybenzoxazine Films
The surface free energy of polybenzoxazines was studied by the contact angle measurements. These four polybenzoxazine films were prepared by curing at different temperature for 1 h. Then, their surface free energies were evaluated by OWRK mode upon Owens’s three-liquid method. The surface free energies of the benzoxazine monomer films were measured firstly (Table 2).
 Table 2.
Contact angles and surface free energies of P-am, P-m, P-b and P-da benzoxazine monomer films
Table 2.
Contact angles and surface free energies of P-am, P-m, P-b and P-da benzoxazine monomer films
Benzoxazine monomer Contact angle (°) Surf. free energy K(mJ·m-2)T Water Ethylene glycol Diiodomethane P-am 74.1 39.0 13.9 45.93 P-m 75.7 61.9 13.1 36.40 P-b 96.0 74.1 71.8 21.71 P-da 80.2 66.2 17.9 39.83 Table 2. Contact angles and surface free energies of P-am, P-m, P-b and P-da benzoxazine monomer filmsSubsequently, the surface free energies of the polybenzoxazine films are shown in Table 3.
 Table 3.
Contact angles and surface free energies of poly(P-am), poly(P-m), poly(P-b) and poly(P-da) benzoxazine films cured at different temperatures
Table 3.
Contact angles and surface free energies of poly(P-am), poly(P-m), poly(P-b) and poly(P-da) benzoxazine films cured at different temperatures
Benzoxazine monomer Curing temp. (℃) Contact angle (°) Surf. free energy K(mJ·m-2)T Water Ethylene glycol Diiodomethane P-am 120 100.3 77.5 66.6 23.11 150 103.7 80.9 68.5 22.10 180 107.4 89.5 77.5 16.74 190 106.7 89.8 78.6 16.13 200 107.0 89.2 75.1 17.53 210 106.9 91.0 80.5 15.15 P-m 120 90.2 66.2 17.9 35.83 150 97.2 72.3 51.6 29.71 180 103.8 81.5 72.5 20.39 190 104.3 83.0 65.7 22.48 200 105.3 85.8 66.7 21.13 210 105.4 89.2 75.8 16.89 P-b 120 97.5 79.9 72.9 19.80 150 104.1 86.2 80.5 16.31 180 101.5 79.4 78.4 18.47 190 99.1 79.3 78.4 18.42 200 92.7 73.4 80.8 19.64 210 94.0 78.4 78.1 19.01 P-da 120 94.2 49.5 36.9 30.63 150 97.2 72.3 51.6 29.71 180 103.8 81.5 62.5 24.39 190 104.3 83.0 65.7 22.48 200 105.3 85.8 66.7 21.13 210 105.5 84.7 61.8 23.76 Table 3. Contact angles and surface free energies of poly(P-am), poly(P-m), poly(P-b) and poly(P-da) benzoxazine films cured at different temperaturesAs shown in Table 3, the lowest surface free energies of poly(P-am), poly(P-m), poly(P-b), and poly(P-da) films were 15.15, 16.89, 16.31 and 21.13 mJ·m-2 respectively, which were cured at 210, 210, 150 and 200℃ for 1 h. In order to compare the surface properties of the four polybenzoxazine films, the surface free energies of these films cured at 200℃ for different time (0.5-16 h) were also measured, and the results are shown in Table 4.
 Table 4.
Contact angles and surface free energies of poly(P-am), poly(P-m), poly(P-b), and poly(P-da) films cured at 200℃ for different time
Table 4.
Contact angles and surface free energies of poly(P-am), poly(P-m), poly(P-b), and poly(P-da) films cured at 200℃ for different time
Benzoxazine monomer Curing time (h) Contact angle (°) Surf. free energy K(mJ·m-2)T Water Ethylene glycol Diiodomethane P-am K1/2T 101.5 77.4 51.4 29.20 1 104.2 82.4 69.4 19.23 2 104.0 83.8 70.1 19.80 3 104.9 87.1 70.7 18.99 4 105.3 89.0 72.1 17.95 8 104.5 88.2 74.5 17.44 16 90.6 81.1 67.3 20.89 P-m K1/2T 104.9 82.1 63.8 23.87 1 105.0 83.5 66.9 22.08 2 102.1 81.4 69.1 21.22 3 96.5 84.0 70.8 18.94 4 94.1 85.7 71.4 18.46 8 105.2 89.7 73.2 17.32 16 103.0 86.4 68.2 19.51 P-b K1/2T 101.9 79.0 48.4 29.27 1 103.5 80.4 67.6 22.11 2 103.8 81.6 68.5 21.86 3 104.0 81.2 70.7 21.22 4 96.6 80.8 70.8 20.03 8 92.8 79.9 74.1 19.65 16 90.8 76.6 75.7 20.40 P-da K1/2T 102.6 80.0 68.0 22.29 1 97.6 78.7 68.6 21.47 2 86.1 74.7 68.0 23.22 3 83.0 65.3 71.3 25.40 4 78.1 71.4 71.3 26.25 8 67.9 70.9 69.7 33.10 16 61.7 72.4 62.6 38.80 Table 4. Contact angles and surface free energies of poly(P-am), poly(P-m), poly(P-b), and poly(P-da) films cured at 200℃ for different timeAs shown in Table 4, the surface free energies of polybenzoxazine films decreased gradually and then increased upon increasing the curing time from 0.5 to 16 h. The lowest surface free energies of poly(P-am), poly(P-m), poly(P-b), and poly(P-da) films were 17.44, 17.32, 19.65 and 21.47 mJ·m-2 respectively, which were cured for 8, 8, 4 and 1 h. The curing time of poly(P-am), poly(P-m), and poly(P-b) films to get the lowest surface free energies were much longer than that of poly(P-da). Compared to the H, CH3, and (CH2)3CH3, the long alkyl chain (CH2)11CH3 had larger steric hindrance, which led to the fast transformation from intra-to intermolecular hydrogen bonds during the curing process. Furthermore, the surface free energies of poly(P-m), poly(P-b), and poly(P-da) films increased with increasing alkyl chain length. As mentioned in the literature[46], the increasing of the alkyl chain length could result in the increasing of intramolecular hydrogen bond length, which was conducive to form the intermolecular hydrogen bond. Thus, the proportion of intermolecular hydrogen bonds increased, and finally the surface free energy increased.
Hydrogen Bonding Network Analysis of Polybenzoxazines
The transformation between the intermolecular hydrogen bonds and the intramolecular ones of the polybenzoxazine materials could lead to the low surface free energy[43, 44]. Especially for the intramolecular hydrogen bonds[14, 39, 40, 52], it contributes much to the low surface free energy. Normally, there are several kinds of H-bonded species in the polybenzoxazine system[52-54], including―OH…N intramolecular hydrogen bonding (3115 cm-1), ―OH…O intermolecular hydrogen bonding (3366 cm-1), and O-…H+N intramolecular hydrogen bonding (between 2500 and 3300 cm-1). FTIR spectra of poly(P-am), poly(P-m), poly(P-b), and poly(P-da) cured for different time were compared for investigating their hydrogen bonding network. Here we took poly(P-b) cured at 200℃ for example (Fig. 4).
In the range of 500 cm-1 to 900 cm-1 (Fig. 4a), the characteristic absorption of the benzoxazine structure at 929 cm-1 disappeared after curing for 1 h. Meanwhile, the absorbance of the band at 1636 cm-1 increased gradually, which was belonged to free Schiff base (C=N)[22]. The polybenzoxazine structure could be thermally degraded at 200℃, and the generated free Schiff bases and the H-Bonded Schiff bases became the terminated group or the defect structure of the polybenzoxazine.
Figure 4(b) displays the changes of the hydroxyl stretching frequency. The O-…H+N intramolecular hydrogen bonding of the poly(P-b) around 2750 cm-1 appeared during the curing process from 0.5 h to 16 h. And it could be found the proportion of intramolecular H-bond (appeared at 3115 cm-1 and 2750 cm-1) was substantially constant after the ring-opening reaction finished (cured about 0.5 h), and the proportion of intermolecular hydrogen bond (appeared at 3368 cm-1) changed continuously. To systematically study the changes of intermolecular H-bond with curing time, curve-resolving of FTIR spectra for the corresponding frequencies of each H-bonded species was performed. The results of curve fitting for the FTIR spectra are shown in Fig. 5.
The OH…O intermolecular H-bonds of poly(P-b) appeared at 3368 cm-1. Upon increasing the curing time, the fraction of these intermolecular H-bonds increased continually. Figure 6 shows the fraction of intermolecular hydrogen bonding and surface free energies of poly(P-m), poly(P-b), and poly(P-da). Combining the analyses of the FTIR spectra (Fig. 5a) and the surface free energies (Fig. 6b), it is demonstrated that after the lowest surface free energy, the enhanced intermolecular hydrogen bonding led to the increasing in the surface free energy.
The similar research was also done for the poly(P-am), and the results are shown in Fig. 7. The characteristic absorption of the benzoxazine structure at 960 cm-1 disappeared after curing for 0.5 h. Meanwhile, the absorbance of the free Schiff base (C=N) bond at 1612 cm-1 increased gradually. The proportion of intermolecular hydrogen bond (appeared at 3359 cm-1) changed continuously. Similarly, the FTIR spectra curve-resolving of poly(P-am) with respect to the corresponding frequencies of each hydrogen-bonded species was also performed. As shown in Figs. 7(c) and 7(d), upon the increasing of the curing time from 0.5 h to 16 h, the fraction of intermolecular hydrogen bonds increased initially and then decreased, while the surface free energy was opposite. It may safely draw the following conclusion that strong intermolecular hydrogen bonding decreased the surface free energies of poly(P-am) films, which was inconsistent with the previous studies[39, 40].
The relationships of the fraction of intermolecular hydrogen bonding and the surface free energies of P-m, P-b and P-da polybenzoxazines were similar but different to that of the P-am polybenzoxazine. This could be due to their different mechanisms of transformation between intramolecular hydrogen bonding and intermolecular hydrogen bonding. As shown in Scheme 3, when the N-substituents were CH3, (CH2)3CH3 and (CH2)11CH3, there were strong intramolecular hydrogen bonds between―OH and N―Y. So a stable six-membered ring was formed. Then the C―C bond cleavage formed Mannich base.
But when the N-substituent was H, there might be―NH…OH―intramolecular hydrogen bond existed besides the above one in the polymer (Scheme 4). So in the transformation process of intramolecular to intermolecular hydrogen bond, there would appear the―NH…OH―intramolecular hydrogen bond. But the process shown in Scheme 3 was still dominated, that was to say strong intermolecular hydrogen bond would increase the surface free energy. However, the appearance of―NH…OH―intramolecular hydrogen bond made the fraction of intermolecular hydrogen bond decrease, thus led to such a phenomenon that the surface free energy increased with the fraction of intermolecular hydrogen bond decreased (Figs. 7c and 7d) after the lowest surface free energy.
As discussed above, the surface free energy was not only affected by the hydrogen bond network but also the N-substituted alkyl. After detailed studying for curing conditions of these polybenzoxazine films, it could be concluded that they were all low surface free energy materials. In comparison, the surface free energy of poly(P-da) was higher than that of others, probably because the long alkyl chain was entangled and curled during curing and couldn’t stretch on the surface.
Preparation of the Polybenzoxazine Films
The polybenzoxazine films were prepared by heat curing (Scheme 2). The benzoxazine monomer films were cured at a certain temperature for a certain time to obtain the polymer films. In this work, the curing temperature was 120-240℃, and the curing time was 0.5-16 h.
Figure 3 shows the FTIR spectra of benzoxazine monomers and polymers from 2000 cm-1 to 500 cm-1. As shown in Fig. 3, the absorption peak of benzoxazine ring at 960 cm-1 (930, 929 and 931 cm-1) disappeared after curing at 200℃ for 1 h. Meantime, the absorption peak at 1611 cm-1 (1638, 1636 and 1633 cm-1) raised on behalf of the characteristic absorption peak of the Schiff base. The results illustrated that the Schiff base had been formed with the ring-open reaction.
CONCLUSIONS
A series of mono-functional benzoxazine monomers with different N-substituents were synthesized and characterized. The polybenzoxazine films were prepared by one-step curing method. Their surface free energies were calculated, and the lowest surface free energies of poly(P-am), poly(P-m), poly(P-b), and poly(P-da) films were 15.15, 16.89, 16.31, and 21.13 mJ·m-2, respectively.
The hydrogen bonding network of polybenzoxazines curing at 200℃ for different time was investigated by analyzing the curve fitting for FTIR spectra. The results showed that the surface free energies of poly(P-m), poly(P-b), and poly(P-da) would increase continuously with the fraction of intermolecular hydrogen bonds increasing after reaching the lowest surface free energies, but poly(p-am) showed different performance. That was because the N-substitute would also affect the surface free energy besides the intermolecular hydrogen bonds. After further study for curing conditions of these polybenzoxazine films, it could be concluded that they were all low surface free energy materials, which indicated their potential applications in the aviation, bio-coatings, and environment protection fields.
-
-
[1]
Ciardelli, F., Aglietto, M., Montagnini di Mirabello, L., Passaglia, E., Giancristoforo, S., Castelvetro, V. and Ruggeri, G., Prog. Org. Coat., 1997, 32:43 doi: 10.1016/S0300-9440(97)00063-5
-
[2]
Klinger, L. and Griffith, J.R., J. Mater. Res., 1987, 2:876 doi: 10.1557/JMR.1987.0876
-
[3]
Cheng, Z., Feng, L. and Jiang, L., Adv. Funct. Mater., 2008, 18:3219 doi: 10.1002/adfm.v18:20
-
[4]
Matsumoto, Y., Yoshida, K. and Ishida, M., Sens. Actuat. Phys., 1998, 66:308 doi: 10.1016/S0924-4247(97)01763-9
-
[5]
Lindner, E., Biofouling, 1992, 6:193 doi: 10.1080/08927019209386222
-
[6]
Zhou, C., Lu, X., Xin, Z., Liu, J. and Zhang, Y., Corros. Sci., 2014, 80:269 doi: 10.1016/j.corsci.2013.11.042
-
[7]
Zhou, C., Lu, X., Xin, Z. and Liu, J., Corros. Sci., 2013, 70:145 doi: 10.1016/j.corsci.2013.01.023
-
[8]
Meng, F., Ishida, H. and Liu, X., RSC Adv., 2014, 4:9471 doi: 10.1039/c3ra47345g
-
[9]
Zhang, T., Fang, Z. and Peng, M., Chinese J. Polym. Sci., 2013, 31(10):1359 doi: 10.1007/s10118-013-1313-9
-
[10]
Zhang, W., Lu, X., Xin, Z., Zhou, C. and Liu, J., RSC Adv., 2015, 5:55513 doi: 10.1039/C5RA06410D
-
[11]
Ishida, H. and Allen, D.J., J. Polym. Sci., Part B:Polym. Phys., 1996, 34:1019 doi: 10.1002/(ISSN)1099-0488
-
[12]
Wirasate, S., Dhumrongvaraporn, S., Allen, D.J. and Ishida, H., J. Appl. Polym. Sci., 1998, 70:1299 doi: 10.1002/(ISSN)1097-4628
-
[13]
Fu, Z., Liu, H., Cai, H., Liu, X., Ying, G., Xu, K. and Chen, M., Polym. Eng. Sci., 2012, 52:2473 doi: 10.1002/pen.v52.11
-
[14]
Qu, L. and Xin, Z., Langmuir, 2011, 27:8365 doi: 10.1021/la200073v
-
[15]
Liu, J., Lu, X., Xin, Z. and Zhou, C., Langmuir, 2013, 29:411 doi: 10.1021/la303730m
-
[16]
Takeichi, T., Thongpradith, S. and Kawauchi, T., Molecules, 2015, 20:6488 doi: 10.3390/molecules20046488
-
[17]
Rao, B.S. and Palanisamy, A., React. Funct. Polym., 2011, 71:148 doi: 10.1016/j.reactfunctpolym.2010.11.025
-
[18]
Chutayothin, P. and Ishida, H., Macromolecules, 2010, 43:4562 doi: 10.1021/ma901743h
-
[19]
Qi, S., Han, G., Wang, H., Li, N., Jiang, S. and Lu, Y., Chinese J. Polym. Sci., 2015, 33(11):1606 doi: 10.1007/s10118-015-1712-1
-
[20]
Kiskan, B., Koz, B. and Yagci, Y., J. Polym. Sci., Part A:Polym. Chem., 2009, 47:6955 doi: 10.1002/pola.v47:24
-
[21]
Tuzun, A., Kiskan, B., Alemdar, N., Erciyes, A.T. and Yagci, Y., J. Polym. Sci., Part A:Polym. Chem., 2010, 48:4279 doi: 10.1002/pola.24215
-
[22]
Zhang, T., Men, W. and Liu, Y., Chinese J. Polym. Sci., 2012, 30(2):250 doi: 10.1007/s10118-012-1115-5
-
[23]
Jin, L., Agag, T., Yagci, Y. and Ishida, H., Macromolecules, 2011, 44:767 doi: 10.1021/ma102351a
-
[24]
Koz, B., Kiskan, B. and Yagci, Y., Polym. Bull., 2011, 66:165 doi: 10.1007/s00289-010-0261-6
-
[25]
Agag, T., Akelah, A., Rehab, A. and Mostafa, S., Polym. Int., 2012, 61:124
-
[26]
Sudo, A., Du, L.C., Hirayama, S. and Endo, T., J. Polym. Sci., Part A:Polym. Chem., 2010, 48:2777 doi: 10.1002/pola.v48:13
-
[27]
Koz, B., Kiskan, B. and Yagci, Y., Polym. Bull., 2010, 66:165
-
[28]
Yang, C.C., Lin, Y.C., Wang, P.I., Liaw, D.J. and Kuo, S.W., Polymer, 2014, 55:2044 doi: 10.1016/j.polymer.2014.02.061
-
[29]
Liu, J., Lu, X., Xin, Z. and Zhou, C., Appl. Surf. Sci., 2015, 353:1137 doi: 10.1016/j.apsusc.2015.06.177
-
[30]
Selvi, M., Devaraju, S., Sethuraman, K., Revathi, R. and Alagar, M., Chinese J. Polym. Sci., 2014, 32(8):1086 doi: 10.1007/s10118-014-1479-9
-
[31]
Wang, C.F., Hung, S.W., Kuo, S.W. and Chang, C.J., Appl. Surf. Sci., 2014, 320:658 doi: 10.1016/j.apsusc.2014.09.122
-
[32]
Wang, S., Li, W.C., Zhang, L., Jin, Z.Y. and Lu, A.H., J. Mater. Chem. A., 2014, 2:4406 doi: 10.1039/c3ta15065h
-
[33]
Ertas, Y. and Uyar, T., Polymer, 2014, 55:556 doi: 10.1016/j.polymer.2013.12.018
-
[34]
Raicopol, M., Bălănucă, B., Sliozberg, K., Schlüter, B., Gârea, S. A., Chira, N., Schuhmann, W. and Andronescu, C., Corros. Sci., 2015, 37:329
-
[35]
Ruiz, C.J.Z., Szczurek, A., de Yuso Arisa, A.M., Ronda, J.C., Cádiz, V., Fierro, V. and Celzard, A., Carbon., 2015, 95:919 doi: 10.1016/j.carbon.2015.09.012
-
[36]
Kim, H.D. and Ishida, H., Macromol. Symp., 2003, 195:123 doi: 10.1002/masy.200390113
-
[37]
Goward, G.R., Sebastiani, D., Schnell, I., Spiess, H.W., Kim, H.D. and Ishida, H., J. Am. Chem. Soc., 2003, 125:5792 doi: 10.1021/ja029059r
-
[38]
Kim, H.D. and Ishida, H., J. Phys. Chem. A., 2002, 106:3271 doi: 10.1021/jp010606p
-
[39]
Wang, C.F., Su, Y.C., Kuo, S.W., Huang, C.F., Sheen, Y.C. and Chang, F.C., Angew. Chem., Int. Ed., 2006, 45:2248 doi: 10.1002/(ISSN)1521-3773
-
[40]
Dong, H., Xin, Z., Lu, X. and Lv, Y., Polymer, 2011, 52:1092 doi: 10.1016/j.polymer.2011.01.009
-
[41]
Stachewicz, U., Li, S., Bilotti, E. and Barber, A.H., Appl. Phys. Lett., 2012, 100:94
-
[42]
Liao, C.S., Wang, C.F., Lin, H.C., Chou, H.Y. and Chang, F.C., Langmuir., 2009, 25:3359 doi: 10.1021/la900176c
-
[43]
Kuo, S.W., Wu, Y.C., Wang, C.F. and Jeong, K.U., J. Phys. Chem. C., 2009, 113:20666 doi: 10.1021/jp9059642
-
[44]
Lin, H.C., Wang, C.F., Kuo, S.W., Tung, P.H., Huang, C.F., Lin, C.H. and Chang, F.C., J. Phys. Chem. B., 2007, 111:3404 doi: 10.1021/jp067909+
-
[45]
Kim, H.D. and Ishida, H., Macromolecules, 2003, 36:8320 doi: 10.1021/ma030108+
-
[46]
Ishida, H. and Low, H.Y., Macromolecules, 1997, 30:1099 doi: 10.1021/ma960539a
-
[47]
Laobuthee, A., Chirachanchai, S., Ishida, H. and Tashiro, K., J. Am. Chem. Soc., 2001, 123:9947 doi: 10.1021/ja004048o
-
[48]
Wang, Y.X. and Ishida, H., J. Appl. Polym. Sci., 2002, 86:2953 doi: 10.1002/(ISSN)1097-4628
-
[49]
Rao, B. and Palanisamy, A., React. Funct. Polym., 2011, 71:148 doi: 10.1016/j.reactfunctpolym.2010.11.025
-
[50]
Chutayothin, P. and Ishida, H., Polymer, 2011, 52:3897 doi: 10.1016/j.polymer.2011.07.006
-
[51]
Owens, D.K. and Wendt, R., J. Appl. Polym. Sci., 1969, 13:1741 doi: 10.1002/app.1969.070130815
-
[52]
Ma, K.X. and Chung, T.S., J. Phys. Chem. B., 2001, 105:4145 doi: 10.1021/jp003103c
-
[53]
Kim, H.D. and Ishida, H., J. Phys. Chem. A., 2002, 106:3271 doi: 10.1021/jp010606p
-
[54]
Goward, G.R., Sebastiani, D., Schnell, I., Spiess, H.W., Kim, H.D. and Ishida, H., J. Am. Chem. Soc., 2003, 125:5792 doi: 10.1021/ja029059r
-
[1]
-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 0
- 文章访问数: 785
- HTML全文浏览量: 8












 下载:
下载:

