Browse Articles
Call for papers
The Chinese Journal of Structural Chemistry, founded in 1982 by Prof. Jiaxi Lu, is an international peer-reviewed journal published in English. It publishes original research works about the structure and property of matter, including but not limited to coordination chemistry, organometallic chemistry, catalysis, energy, nanomaterial, theory/computation, structural characterization, pharmacy and life science. Published monthly by Fujian Institute of Research on the Structure of Matter, CAS, in the form of Articles, Communications, Reviews, Perspectives, and News & Views. The journal is published twelve issues a year by Fujian Institute of Research on the Structure of Matter, CAS and is available online:
https://www.sciencedirect.com/journal/chinese-journal-of-structural-chemistry
Contact information:
E-mail: cjsc@fjirsm.ac.cn; Tel: +86-591-63173769
Display Method: |
While ultrasmooth 2D c-MOF films have demonstrated enhanced charge mobility within the conjugated plane, it is equally imperative to enhance the interlayer pathways for out-of-plane charge transport in three dimensions. This can be achieved through the strategic expansion of conjugated systems, optimization of stacking models, and modification of functional groups. Moreover, there is an urgent need to develop alternative and simpler synthesis methods for defect-free single-crystal thin films, addressing the challenges of low yield and harsh synthesis conditions associated with chemical vapor deposition (CVD) and subsequent separation and washing processes involving gallium. Finally, the integration of density functional theory calculations with machine learning approaches could be a great opportunity in predicting and synthesizing the next generation of conductive 2D c-MOFs.
This study outlines the design principles and potential applications of dynamic 2D COFs, emphasizing the crucial roles of rigid columns and flexible bridges. It serves as a valuable reference for understanding and creating novel COFs with tunable properties. Future research can utilize the design principle of ‘wine rack’ frames to achieve lateral offset of π-stacked columns by introducing branched chains with strong spatial positional resistance at COF nodes and connecting them with flexible connectors, thus ensuring the robustness and flexibility of the COF structure to enable dynamic structural transformations in response to external stimuli. This structural adaptability can be utilized to control pore size, pore volume, and exciton coupling within the material, enabling practical applications of COFs in gas separation, molecular adsorption, and photoelectrocatalysis.
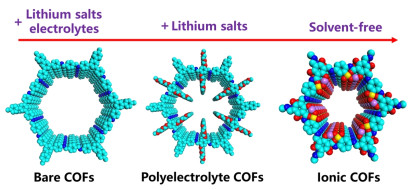
In summary, this research marks a major advancement in the development of medium-temperature PEMFCs. The novel COF ionomer offers solutions to long-standing challenges related to water retention, proton transport, and oxygen mass transport at elevated temperatures. The improved performance of MT PEMFCs using this material opens possibilities for broader applications in the energy sector, particularly in automotive and stationary power generation. This innovation could pave the way for the next generation of PEMFCs, overcoming some of the key obstacles that have limited their application in more demanding applications. Future research should focus on optimizing the synthesis process for large-scale production and exploring the ionomer's performance under a wider range of operating conditions.
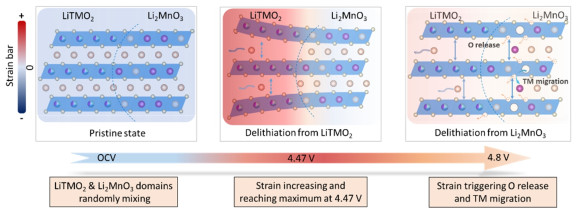
A series of structural characterizations demonstrated that the introduction of Li-ions with over-stoichiometric ratios led to the localized formation of a unique nanophase (S-phase), which stabilized the coplanar Li-ion conformation and enabled lithium superionic conduction. The construction of this face-sharing lithium structure has great application prospects for improving the ionic conductivity of FCC-type oxides. The research in this work deepens the understanding of the mechanism of rapid lithium ion migration in oxide materials and will accelerate the development of new oxide electrolytes for all-solid-state batteries. Although the present materials exhibit almost equal lithium ion conduction with the current hot oxide-based solid electrolyte (such as lithium garnet, lithium peroxide, Na superionic conductor (NASICON) lithium oxide), there are still many challenges to overcome for their potential applications. For example, the preparation conditions of the material are harsh, requiring high temperature quenching at 1050 ℃. Secondly, the o-LISO compounds are sensitive to air and need to be stored in the glove box. Nonetheless, the face-sharing strategy proposed by Chen et al. is inspiring for the exploration of new solid functional materials in structures that were previously overlooked.
Although mimicking protein functions using supramolecular systems remains a daunting challenge, the impressive work by Aprahamian et al. suggests that future efforts may focus on nonequilibrium rather than equilibrium states, and that combining dynamic chemistry with host-guest chemistry is a worthwhile direction for creating more complex and biomimetic smart molecules.
In summary, the fabrication of oxide photoelectrodes are still attractive and challenging filed towards various application. Although some fast preparation methods have been developed, the performance of related oxide photoelectrodes are still relatively low comparing to their maximum theoretical performance. As a perspective on oxide photoelectrodes, we offer suggestions for the fast fabrication of photoelectrodes. First, the formation mechanism of photoelectrodes should be elucidated in details and how to prepare high performance photoelectrodes should be answered. Second, the devices for fast fabrication photoelectrodes should be simplistic and low cost. Furthermore, more effort should be put on developing large scale preparation strategies by fast annealing processes.
Despite these challenges, nano-thermometry possesses great potentialss for highly sensitive, non-contact temperature measurements with sub-microscopic resolution towards in-depth understanding on optimized catalysis. Further technological advances of nano-thermometry in photothermal catalysis could focus on developing generalized, high-precision and in-situ temperature measurement techniques under working conditions to clearly distinguish between thermal and non-thermal contributions, and to deepen understandings on photothermal catalytic reaction mechanisms, which could, in turn, provide important design principles for efficient catalytic systems. In addition, future nano-thermometry could be more integrated and intelligent, incorporating real-time big-data processing and principal-component analysis systems to carry out more accurate and efficient temperature measurement. Finally, the development of nano-thermometry could be combined with multi-spectroscopy technology to provide more comprehensive solutions for catalytic reactions. These developments will help significantly improve the accuracy and reliability of temperature measurement for catalytic reactions.
In conclusion, the synthesis of nine heterometallic Sn-Co/Mn nanoclusters has been achieved through a stepwise self-assem bly approach, utilizing polynuclear oxygen donors as key precur sors. The tBuPO32- ligands play a pivotal role in bridging trinuclear organotin clusters with Co2+/Mn2+ ions. Shielded by O/N/P-con taining ligands, the Co2+ and Mn2+ ions assume distorted tetrahe dral and hexahedral coordination environments, respectively. Lig ands featuring two or three N/P coordination sites facilitate the bridging of multiple [(nBuSn)3(MeO)3(μ3-O)(tBuPO3)3Co] substruc tures, highlighting their structural robustness. Significantly, the dy namic magnetization measurements indicated that (Sn3-Co1)2-1,5-naphyd NC exhibits a slow relaxation behavior of magnetiza tion. This study delves into the intricate self-assembly process of Sn-Co/Mn nanoclusters at the molecular level, offering valuable insights for the development of novel SMMs.
H2O-induced side reactions and dendrite growth occurring at the Zn anode-electrolyte interface (AEI) limit the electrochemical performances of aqueous zinc ion batteries. Herein, methionine (Met) is introduced as an electrolyte additive to solve the above issues by three aspects: Firstly, Met is anchored on Zn anode by amino/methylthio groups to form an H2O-poor AEI, thus increasing the overpotential of hydrogen evolution reaction (HER); secondly, Met serves as a pH buffer to neutralize the HER generated OH-, thereby preventing the formation of by-products (e.g. Zn4SO4(OH)6·xH2O); thirdly, Zn2+ could be captured by carboxyl group of the anchored Met through electrostatic interaction, which promotes the dense and flat Zn deposition. Consequently, the Zn||Zn symmetric cell obtains a long cycle life of 3200 h at 1.0 mA cm-2, 1.0 mAh cm-2, and 1400 h at 5.0 mA cm-2, 5.0 mAh cm-2. Moreover, Zn||VO2 full cell exhibits a capacity retention of 91.0% after operating for 7000 cycles at 5.0 A g-1. This study offers a novel strategy for modulating the interface microenvironment of AEI via integrating the molecular adsorption, pH buffer, and Zn2+ capture strategies to design advanced industrial-oriented batteries.
The investigation of thermal transport properties of materials has become increasingly important in technological applications, including thermal management and energy conversion. Recently, ultrahigh or low thermal conductivity has been reported in nitride, boride, and chalcogenide by different strategies. However, the strategy to design oxide crystals with unique thermal properties is also a challenge. In this work, a new ternary oxide crystal Ga2TeO6 is designed and expected to show high thermal conductivity due to its lone pairs-free octahedra connected along the c-axis by sharing edges. The thermal conductivities of Ga2TeO6 crystal are determined to be 19.2 W m-1 K-1 and 23.9 W m-1 K-1 along the a- and c-axes directions at 323 K, respectively, which are significantly higher than those of most reported oxide crystals. First-principles calculations and crystal structure analyses reveal that the Ga2TeO6 crystal shows high sound velocity and weak lattice anharmonicity due to lone pairs-free octahedra and highly symmetric group arrangement. The results suggest that much attention must be paid to the polyhedron with lone pairs and its arrangement in materials design to balance the functions and thermal properties.
Negatively charged open-framework metal sulfides (NOSs), taking advantages of the characteristics of excellent visible light absorption, easily exchanged cations, and abundant active sites, hold significant promise as highly efficient photocatalysts for hydrogen evolution. However, their applications in photocatalytic hydrogen evolution (PHE) are infrequently documented and the corresponding photocatalytic mechanism has not yet been explored. Herein, we excavated a novel NOS photocatalyst of (Me2NH2)6In10S18 (MIS) with a three-dimensional (3D) structure, and successfully incorporated divalent Co(II) and metal Co(0) into its cavities via the convenient cation exchange-assisted approach to regulate the critical steps of photocatalytic reactions. As the introduced Co(0) allows for more efficient light utilization and adroitly surficial hydrogen desorption, and meanwhile acts as the ‘electron pump’ for rapid charge transfer, Co(0)-modified MIS delivers a surprising PHE activity in the initial stage of photocatalysis. With the prolonging of illumination, metal Co(0) gradually escapes from MIS framework, resulting in the declining of PHE performance. By stark contrast, the incorporated Co(II) can establish a strong interaction with MIS framework, and meanwhile capture photogenerated electrons from MIS to produce Co(0), which constructs a stable photocatalytic system as well as provides additional channels for spatially separating photogenerated carriers. Thus, Co(II)-modified MIS exhibits a robust and highly stable PHE activity of ~4944 μmol/g/h during the long-term photocatalytic reactions, surpassing most of previously reported In-S-framework photocatalysts. This work represents a breakthrough in the study of PHE performance and mechanism of NOS-based photocatalysts, and sheds light on the design of guest confined NOS-based photocatalysts towards high-efficiency solar-to-chemical energy conversion.
As a highly reactive reaction intermediate, surface gallium hydride (Ga-H) has garnered significant attention due to its critical role in various catalytic reactions. However, the detailed experimental characterization of this unique species remains challenging. Recently, we demonstrated that solid-state NMR can be an effective tool for studying surface Ga-H. In this work, we report a comparative solid-state NMR study on H2 activation over different Ga2O3 polymorphs, specifically α-, β- and γ-Ga2O3. 1H solid-state NMR enabled the identification of Ga-H species formed on all the three samples following high-temperature H2 treatment. The characteristic 1H NMR signals of the Ga-H species were resolved using J-coupling-based double-resonance NMR methods, revealing highly similar lineshapes of Ga-H for all the Ga2O3 samples. This suggests potentially similar surface Ga-H configurations among the different Ga2O3 polymorphs. In addition, the local hydrogen environments on the oxide surfaces were further explored using two-dimensional (2D) 1H-1H homonuclear correlation spectra, revealing multiple spatially proximate Ga-H pairs and Ga-H/-OH pairs on different Ga2O3 polymorphs. These findings provide insights into the potential mechanism of H2 dissociation. Overall, this work offers new perspectives on the local structure of surface Ga-H on Ga2O3, and the analytical approach presented here can be further extended to the study of other Ga-based catalysts and other metal hydride species.
Partitioning of actinides from lanthanides is pivotal for advancing nuclear waste management and sustaining nuclear energy development, yet it remains a formidable challenge due to the intricate chemical behavior of these f-block elements. In this study, we introduce 3,6-di-2-pyridyl-1,2,4,5-tetrazine (L1), whose hydrolysis product of pyridine-2-carbox-aldehyde (pyridine-2-carbonyl)-hydrazone (L2) can fractionally crystallize U(VI) ions over Ln(III) cations with high selectivity and efficiency. Through hydrolysis-induced C–N bond cleavage, L2 acts as a tetradentate ligand, coordinating with two UO22+ ions in a planar arrangement to form a zero-dimensional cluster, [(UO2)2(μ3-O)(L2)(CH3COO)]·DMF (U-L2), while lanthanide ions (Ln = La, Pr, Nd, Sm, Eu, Gd, Tb, Yb, and Lu) remain in solution due to their inability to achieve similar coordination. This selective crystallization strategy yields exceptional separation factors between U(VI) and Ln(III), with a separation factor of 756276 between U(VI) and Sm(III), the highest reported to date. Furthermore, this fractional crystallization separation process can be achieved under mild ambient conditions with high separation factors, enabling the development of a rapid, safe, and energy-efficient strategy for once-through separation of high oxidation state actinides from lanthanides.
The advancement in catalysis techniques for sustainable environmental applications, particularly an alternative to the current Haber-Bosch process for NH3, has recently gained widespread attention. Although photocatalytic conversion of N2 to NH3 using solar energy is an eco-friendly method, it has the limitation of low quantum yield. Recently, 2D Bi-based photocatalysts have been an active research topic which exhibit higher visible light absorption than TiO2 and higher stability than MXene. The performance of Bi-based photocatalysts can be enhanced through improved visible light absorption properties by incorporating plasmonic gold nanoparticles while nitrogen adsorption could be enhanced through oxygen vacancy processes. In the present study, we explore the application of 2D nano-sized Bi2O3-x and gold nanoparticles for visible light photo generation of NH3. HRTEM and XPS reveal that the formation of AuNP and nano-sized Bi2O3-x in AuNP/Bi2O3-x heterozygote structure which promotes the charge carrier mobility and charge transport at the interface resulting in a 2.6-fold increase in the photocatalytic activity compared to micro-sized Bi2O3-x with AuNP. The improved photocatalytic performance can be ascribed to significant enhancement of visible light absorption by plasmonic nanoparticles, fast charge transport and mobility (due to sheet morphology) and the N2 activation by oxygen vacancy in AuNP/Bi2O3-x heterozygote. Through a systematic experimental investigation involving catalysts, concentration, pH, and scavengers, the highest photocatalytic performance was achieved with the heterozygote structures of AuNP/n-Bi2O3-x under optimized conditions, yielding 432.5 μmol gcat-1 h-1 of NH3.
Hydrogen peroxide (H2O2) is a crucial, eco-friendly oxidizing agent with a wide range of industrial, environmental, and biomedical applications. Traditional production methods, such as the anthraquinone process, face significant challenges in terms of energy consumption and environmental impact. As a sustainable alternative, photocatalytic H2O2 production, driven by solar energy, has emerged as a promising approach. This review discusses the key advancements in photocatalytic H2O2 synthesis, focusing on overcoming challenges such as charge recombination, selectivity for the two-electron oxygen reduction reaction (2e- ORR), and catalyst stability. Recent innovations in photocatalyst design, including high-entropy materials, single-atom catalysts, and covalent organic frameworks (COFs), have significantly enhanced efficiency and stability. Furthermore, novel strategies for optimizing charge separation, light harvesting, and mass transfer are explored. The integration of artificial intelligence and bioinspired systems holds potential for accelerating progress in this field. This review provides a comprehensive overview of current challenges and cutting-edge solutions, offering valuable insights for the development of scalable, decentralized H2O2 production systems that contribute to a more sustainable future.

 Login In
Login In