Preparation of Dilauric Acid Thiodiglycol Ester and Evaluation of Its Antioxidant Capacity in Polypropylene Resin
Polymer antioxidants, employed as one of the additives to polymers to reduce or inhibit oxidation, play an important role in improving the stability of polymers when they are subjected to photo-and thermo-oxidation during processing and application periods[1, 2]. Polypropylene (PP) is one of the most widely used polymers in many industrial applications, and considerable attention has focused on antioxidants which can prevent PP from oxidative degra-dation[3~6]. Oxidation of polymers generally arises from the formation of peroxy free radicals leading to undesirable reactions which usually cause chain scission, crosslinking, and discoloration, as well as a decline in mechanical and physical properties[7]. So substances which act as free radical scavengers or react with hydroperoxide groups could be used for the stabilization of polymers[8]. A variety of compounds, including sterically hindered amines (e.g. Tinuvin, Omnistab, SABO), phenols (e. g. 1010, 1076, HP-136), phosphites, phosphonites and sulfur derivatives, have been used as polymer antioxi-dants[9~11]. According to their mode of operation, antioxidants can be divided into primary and secondary[12]. Primary antioxidants such as hindered phenols inhibit oxidation through donation of a hydrogen atom[13]. Thioester-and thioether-type antioxidants, which exhibit excellent peroxides decompo-sition capacity and color stabilizing ability, are secondary antioxidants[14]. Positive synergism is generally present when they are used together with phenols and aromatic amines, thus thioester- and thioether-type antioxidants are widely employed as additives in polystyrene, polyethylene, PP and rubber[15].
Thiodiglycol is the main by-product in the prod-ucing of β-mercaptoethanol. The residue is responsible for some environmental problems in the industry, and diminishing its environmental impact has been the subject of an increasing concern in recent years[16, 17]. Interestingly, while the use of dilauryl thiodipropionate (DLTP) and distearyl thiodipropionate (DSTP) as antioxidants in polymer industry is well known[18], the investigations of thiodiglycol esters, the principal derivative of thiodiglycol, as antioxidants are scarce. Due to the mechanisms of the antioxidant actions of thiodipro-pionate esters[19, 20] and the structural similarity between DLTP and thiodiglycol esters, it is possible that thiodiglycol dicarboxylic acid esters show antioxidant activity and thus be a kind of possible polymer antioxidants. In our previous work, dimyristic acid thiodiglycol ester was found to exhibit good antioxidant activity in PP[21].
The aim of the present work is to propose a way to utilize thiodiglycol as a source for preparing dilauric acid thiodiglycol ester (DATE) for the possible use as polymer antioxidant. To this purpose, novel DATE was synthesized from thiodiglycol and its antioxidant efficiency in PP was evaluated as well as its effect was compared with other commercially available antioxidants applied in the polymer industry by the measurement of melt flow index (MFI), elongation at break, impact strength and tensile strength of PP blended with antioxidants.
1.1 Materials and Methods
Thiodiglycol with a purity of 98% after distillation were provided by Maoming Fengyuan Fine Chemical Technology Co., Ltd., China. Antioxidant 1010, 1076, 168 and DSTP, food-grade, were purchased from CIBA company, Switzerland. PP powder was purchased from Maoming Branch Corp. of SINOPEC, China. All the other chemicals supplied by Guangda, China, were used as received without further purification.
1H NMR and 13C NMR spectra were recorded in CDCl3 on Bruker DRX-400 (400MHz) spectrometers. EI mass spectroscopy was performed with ion source temperature of 230℃. GC-MS was performed on a Frinnigan TRACE-DSQ spectrometer equipped with quartz HP-5 column (30 m by 0.25 mm ID by 0.2 mm film thickness) using helium as carrier gas. The injector temperature was 250℃ and the column temperature was programmed to rise from an initial temperature of 60℃, maintained for 1 min, to 280℃ at 25℃/min. IR spectra were obtained as potassium bromide films using a Nicolet 760 FT-IP spectrometer. Melting point (mp) was taken on sample in open capillary tube and was uncorrected.
1.2 Synthesis and characterization of DATE
Thiodiglycol (19.2g, 0.157mol), lauric acid (24.1 g, 0.12 mol) and p-TsOH (0.49g, 2% by weight of lauric acid) were mixed with 40 mL of xylene. The mixture was refluxed for 3h until no water was carried out by xylene. The reaction mixture was cooled to room temperature and washed with water 3 times. The organic layer was separated and 40 mL of methanol was added. The mixture was heated and the upper layer was separated. The procedure was repeated 3 times and the combined upper layer was allowed to stand at -15℃ overnight. The crude product was filtered and further purified by recrystallization in methanol which afforded DATE[(CH3(CH2)8CH2CH2COOCH2CH2)2S] as a white solid. m.p. 48~50℃; 1H NMR (400MHz, CDCl3) δ: 4.19~4.23 (m, 2H, -CH2O-), 2.73~2.79 (m, 2H, -CH2S-), 2.29 (t, J =5.7Hz, 2H, -CH2CO-), 1.59 (t, J=5.4Hz, 2H, -CH2CH2CO-), 1.23~1.26 (m, 16H, -(CH2)8-), 0.85 (t, J =5.1Hz, 3H, -CH3); 13C NMR (101MHz, CDCl3) δ: 173.8, 63.4, 32.0, 30.7, 29.7, 29.6, 29.5, 29.4, 29.3, 25.0, 24.9, 22.8, 14.3; IR (KBr film) υ/cm-1: 2917, 2849, 1736, 1460, 1177; MS-EI, m/z: 227, 86, 69, 57, 43.
In the FT-IR spectrum, the absorptions at 2917 cm-1 and 2849 cm-1 are assigned to the C—H asymmetric and symmetric stretching vibration. The strong band at 1738 cm-1 is the characteristic stretching of CO. The absorption around 1460 cm-1 is attributed to C—H bending, and the absorption around 1177 cm-1 is assigned to C—O stretches.
To a flask containing 10g of PP powder was added 0.01g of the antioxidant (0.1% by weight) and cyclohexane. The mixture was stirred and heated with hot water bath until the cyclohexane was evaporated completely. The formed blend of dry PP powder and antioxidant was cooled to room temperature and was added into a XNR-400 melt flow indexer which had been preheated at 230℃ for 30min. After heated for 7 min the mixture was extruded into thin films under a pressure of 3.04 kg·cm-2. The film was cut down every 30 minutes and was measured by its weight. The average weight of the films extruded in 10 min was employed as a measurement of melt flow index.
1.4 Characterization of mechanical properties
Thin wall sheets of pure PP or PP blended with antioxidant (0.1% by weight) were prepared via injection molding according to GB/T 1043-1993 and were allowed to stand at room temperature for 24h before they were used. Specimens for mechanical property tests were cut from these sheets. The elongation at break and the tensile strength of the specimens were determined at room temperature using an Instron 4465 universal testing machine according to ISO-527. The impact strength of the specimens was carried out according to ISO-179 using an I200XJU-2.75 impact tester at room temperature. Each reported result is an average of 5 measurements.
2.1 Effect of catalyst on the synthesis of DATE
Due to the simple procedure and readily available precursors, acid-catalyzed direct esterif-ication of alcohols and acids has been well established as a method for the formation of esters. The method was employed in the synthesis of DATE in this work. In our preliminary studies, we scree-ned a range of catalysts, including H2SO4, H3PO4, Ti(O-n-Bu)4, and p-TsOH, for the production of DATE in xylene at 140℃ for 1 hour, under a molar ratio of thiodiglycol to acid as 2:1. Some results from that study are summarized in Fig. 1.
It was concluded from Fig. 1 that catalyst played an important role in the transformation. Among the applied catalysts, Ti(O-n-Bu)4 had no catalytic activity in the conversion. The mineral acids, H2SO4 and H3PO4, gave moderate to good yields, but the product was in yellowish color. Compared to the catalysts mentioned above, p-TsOH was proved to be an efficient catalyst for the transformation, affording the desired compound with not only lighter color but also in higher yield. Therefore, p-TsOH was selected as catalyst for the further optimization.
2.2 Effect of reaction conditions on esterification of thiodiglycol and lauric acid
Direct esterification often needs removal of water generated in the reaction to shift the equilibrium between reactants and products. Entrainer with high boiling point makes the reaction at a higher temperature and thus has a positive effect on esterifications. However, entrainer with a much higher boiling point than the reactants usually carries away too much starting materials, leading a decrease in the yield of esters. Considering that the starting material had a boiling point of about 140℃, we chose xylene as entrainer for the esterification of thiodiglycol and lauric acid.
To optimize the present reaction, molar ratios of thiodiglycol to lauric acide, loadings of catalyst, and reaction time were screened. Some results were shown in Tab. 1.
表 1
Table 1
表 1(Table 1)
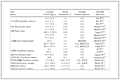
Table 1 Reaction conditions on p-TsOH catalyzed preparation of DATE
表 1 对甲苯磺酸催化制备DATE反应条件的影响
|
Entry |
Molar ratio of
thiodiglycol
to lauric acid |
Loading of
catalyst/%a |
Reaction
time/h |
Yield/%b |
|
1 |
2:1 |
1 |
1 |
82 |
|
2 |
2:1.5 |
1 |
1 |
84 |
|
3 |
1:1 |
1 |
1 |
79 |
|
4 |
2:1.5 |
2 |
1 |
87 |
|
5 |
2:1.5 |
4 |
1 |
80 |
|
6 |
2:1.5 |
2 |
3 |
94 |
|
7 |
2:1.5 |
2 |
4 |
89 |
| a By weight of lauric acid; b Isolated yield. |
|
Table 1 Reaction conditions on p-TsOH catalyzed preparation of DATE
表 1 对甲苯磺酸催化制备DATE反应条件的影响
|
Lowering the molar ratio of thiodiglycol to lauric acid resulted in a little higher yield (entry 2, Tab. 1),whereas further lowering it to 1:1 ratio led to a much lower yield. Increasing the loading of catalyst to 2% by weight of lauric acid, an 87% isolated yield of DATE was obtained (entry 4, Tab. 1). However, the yield decreased when further increasing the loading of catalyst was conducted. The decrease could be ascribed to the fact that more catalyst accelerated the formation of by-products such as dithiane, linear polymeric ethers and oxathiane[22]. It was proved that 3 h were adequate for completion of the reaction, giving DATE in 94% yields.
2.3 Effects of different antioxidants on the MFI and mechanical properties of PP
MFI is one of the most efficient measurements to evaluate the relative molecular weight of polymers, making it an attractive method to study antioxidant activities. Oxidation generally occurs during the processing of polymers, leading to the decrease in polymer molecular weight and loss of mechanical properties. The decrease of molecular weight of PP is indicated by the variation of its MFI before and after addition of antioxidants.
With it in hand, DATE is applied in PP powder and its antioxidant capacity is studied by MFI measurement. Some other commercially available antioxidants such as 1010 (tetrakis[methylene-3-(3′, 5′-di-tert-butyl-4′-hydroxyphenyl) propionate] methane), 1076 (benzenepropanoic acid, 3, 5-bis(1, 1-dimethylethyl)-4-hydroxy-, octadecyl ester), 168 (tris(2, 4-ditert-butylphenyl) phosphate) and DSTP were also applied in the experiment to compare with DATE. The results were summarized in Fig. 2.
As shown in Fig. 2, the MFI of PP containing DATE is obviously lower than PP without any antioxidant. The antioxidant capacity of DATE is much better than commercially available antioxidant 1010, 168 and DSTP and is only slightly lower than antioxidant 1076.
表 2
Table 2
表 2(Table 2)

Table 2 Comparison of commercially available antioxidants with DATE on mechanical properties of PP
表 2 DATE与其他商品化抗氧化剂对PP机械性能影响的比较
|
Antioxidant |
Loading
/% |
Elongation
at break
/% |
Tensile
strength
/MPa |
Impact
strength
/kJ·m-2
|
|
— |
— |
56.6 |
34.3 |
78.3 |
|
DATE |
0.1 |
112.2 |
39.8 |
121.0 |
|
1010 |
0.1 |
104.4 |
38.1 |
104.4 |
|
1076 |
0.1 |
ND |
ND |
ND |
|
168 |
0.1 |
86.3 |
39.0 |
104.6 |
|
DSTP |
0.1 |
ND |
ND |
ND |
| ND: not determined |
|
Table 2 Comparison of commercially available antioxidants with DATE on mechanical properties of PP
表 2 DATE与其他商品化抗氧化剂对PP机械性能影响的比较
|
The detailed elongation at break, tensile strength and impact strength of the PP incorporated with different antioxidants are also given in Tab. 2. According to the data of mechanical properties, PP containing any antioxidant showed substantial improvement in all indexes compared to that of PP without any additives. It is found that PP containing 0.1% of DATE by weight had the highest values of Elongation at break, Tensile strength and impact strength among the antioxidants tested. These results indicate that DATE may be used as an excellent antioxidant for PP.
2.4 Synergism between DATE and other oxidants in contribution to MFI and mechanical properties of PP
It was well known that antioxidants such as phosphites, phosphonites, and thioesters are usually used in combination with phenol or amine antioxidant[23]. DATE contains low-valent sulfur and has a similar molecular structure with DSTP. For this point of view, it was interesting to investigate whether the combination of DATE with 1010 or 168 would improve the antioxidant efficiency. With the loading of combined antioxidants as 0.1% by weight of PP, the decrease in PP molecular weight was measured by MFI method. The results shown in Tab. 3 indicated that DATE/1010 and DATE/168 as combined antioxidants showed much lower MFIs than that of DSTP/1010 and 168/1010. The PP containing the combined antioxidant also showed a slightly better MFI value than that containing onlyDATE.
表 3
Table 3
表 3(Table 3)

Table 3 MFI of PPs containing different combined antioxidants
表 3 不同复配抗氧化剂对PP熔体流动指数的影响
|
Antioxidant |
DATE |
DATE/1010 |
DATE/168 |
DSTP/1010 |
168/1010
|
Loading/%(by
weight of PP) |
0.1 |
0.05/0.05 |
0.05/0.05 |
0.05/0.05 |
0.05/0.05 |
MFI
(g·10min-1) |
3.59 |
3.56 |
3.28 |
3.80 |
4.10 |
|
Table 3 MFI of PPs containing different combined antioxidants
表 3 不同复配抗氧化剂对PP熔体流动指数的影响
|
During our further investigation of the mechanical properties of PPs incorporated with single prepared antioxidant or combined antioxidant, we found that the elongation at break increased to 140.8% from 112.2%, the tensile strength reached 40.2MPa from 39.8MPa, and the impact strength increased to 124.2kJ·m-2 from 121.0kJ·m-2 when DATE and 168 were used as a combined antioxidant, much better than those when it was used alone (Tab. 4).
表 4
Table 4
表 4(Table 4)

Table 4 Improved mechanical properties of PP containing DATE/168
表 4 DATE/168 对PP 机械性能的改进
|
Mechanical property |
Elongation
at break/% |
Tensile
strength/MPa |
Impact strength
/
kJ·m-2 |
|
DATE (0.1 (wt) %) |
112.2 |
39.8 |
121.0 |
DATE/168(0.05(wt)%/
0.05 (wt)%) |
140.8 |
40.2 |
124.2 |
|
Table 4 Improved mechanical properties of PP containing DATE/168
表 4 DATE/168 对PP 机械性能的改进
|
This work was an attempt to employ a new antioxidant based on thiodiglycol derivatives in PP. The optimized conditions for the preparation of dilauric acid thiodiglycol ester were obtained as reaction temperature at 140℃, molar ratio of thiodiglycol to acid at 2:1.5, catalyst loading at 2% by weight of lauric acid and 3 hours of reaction time in the presence of xylene as entrainer. The antioxidant effectiveness of dilauric acid thiodiglycol ester and combination of it with other antioxidants in propylene was studied by measurements of melt flow index, elongation at break, tensile strength and impact strength. The results achieved indicated that dilauric acid thiodiglycol ester is an extremely effective additive in inhibiting PP from oxidation. Positive synergism between dilauric acid thiodiglycol ester and 168 or 1010 was observed. This makes it very interesting from both the industrial and environmental points of view as a useful antioxidant in polymers. Optimization of structure of the thiodiglycol ester to improve antioxidant activity was in progress in our laboratory.
 2016,
Vol. 79
2016,
Vol. 79 Issue (1): 37-42
Issue (1): 37-42 2016,
Vol. 79
2016,
Vol. 79 Issue (1): 37-42
Issue (1): 37-42