Citation:
Wan Jinyu, Liu Yifei. Toxicity Prediction of Organophosphorus Compounds based on QSAR Model[J]. Chemistry,
;2019, 82(10): 926-936.

-
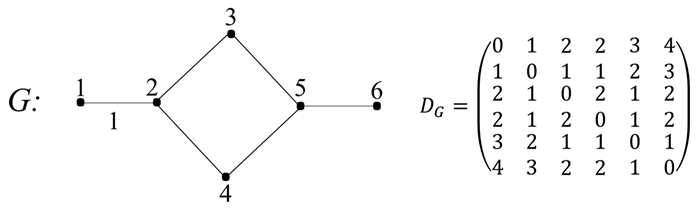
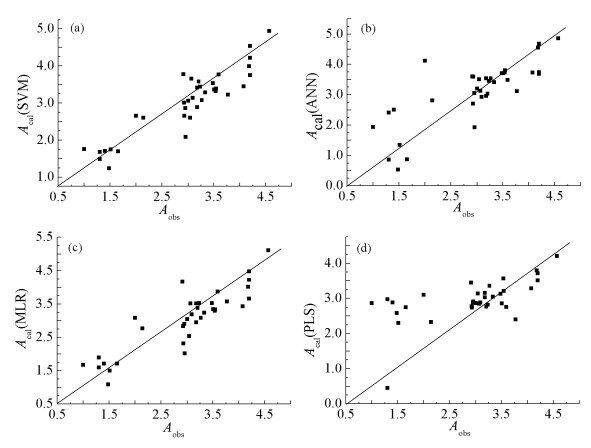
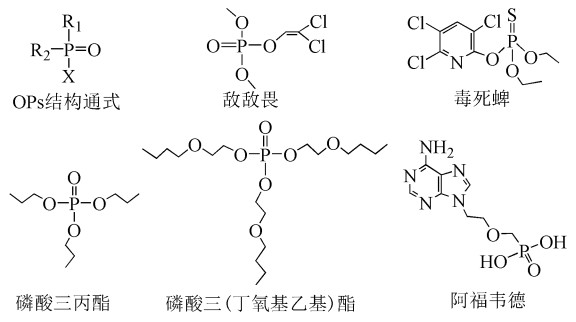
With the wide application of organophosphorus compounds (OPs), they have been detected in an increasing number of environmental media. Most OPs are poisonous, but people often lack fast and effective prediction methods to evaluate the toxicity. In this paper, the toxicity of 36 OPs was predicted by using different QSAR models combined with molecular descriptors by computer with E-Dragon software. Using the backward method as the screening result, root mean square error (RMSE) as evaluation standard, we found that the 14 molecular descriptors have a great influence on the linear kernel function of support vector machine (SVM) model. In the cross-validation results of SVM models, the correlation coefficient between the calculated value and the actual value was 0.913, the root mean square error is 0.388. In the results of external test verification, the average relative error was 9.10%. In addition, we also used multiple linear regression (MLR), artificial neural network (ANN) and partial least squares regression (PLS) models to predict the toxicity of OPs. The cross-validation results showed that the correlation coefficients between the calculated values and the actual values of the three models are 0.878, 0.686 and 0.620, respectively. Therefore, the linear kernel SVM model is an effective method for the toxicity prediction of OPs.
-

-
-
[1]
G W Wang, H Y Chen, Z K Du et al. Sci. Total Environ., 2017, 590~591(15):50~59.
-
[2]
Z K Du, G W Wang, S X Gao et al. Aquat. Toxicol., 2015, 161:25~32.
-
[3]
M Alfonso, R Duran, D Fajardo et al. Neurochem. Int., 2019, 124:130~140.
-
[4]
A Derbalah, R Chidya, W Jadoon et al. J. Environ. Sci., 2019, 79:135~152.
- [5]
-
[6]
J Liu. Appl. Chem. Ind., 2018, 47(12):2706~2710.
- [7]
- [8]
-
[9]
T C Marrs. Pharmacol. Ther., 1993, 58(1):51~66.
-
[10]
K MacPhee-Quigley, P Taylor, S Taylor. J. Biol. Chem., 1985, 260(22):12185~12189.
-
[11]
H Sanderson, P Fauser, M Thomsen et al. J. Hazard. Mater., 2008, 154(1~3):846~857.
-
[12]
R Naven, S Louise-May. Hum. Exp. Toxicol., 2015, 34:1304~1309.
-
[13]
M H Baig, K Ahmad, S Roy et al. Curr. Pharm. Des., 2016, 22:572~581.
-
[14]
M D Segall, C Barber. Drug Discov. Today, 2014, 19(5):688~693.
-
[15]
A Rybinska, A Sosnowska, M Grzonkowska et al. J. Hazard. Mater., 2016, 303:137~144.
-
[16]
L D Cao, P Zhu, Y S Zhao et al. J. Hazard. Mater., 2018, 352(15):17~26.
-
[17]
Y Paukku, G Hill. Int. J. Quantum Chem., 2012, 112(5):1343~1352.
-
[18]
E B A Filho, A A Santos, B G Oliveira. J. Mol. Struct., 2017, 1133:338~347.
-
[19]
-
[20]
H Moriwaki, Y S Tian, N Kawashita et al. J. Cheminform., 2018, 10:4.
-
[21]
I V Tetko, J Gasteiger, R Todeschini et al. J. Comput. Aid. Mol. Des., 2005, 19(6):453~63.
- [22]
- [23]
- [24]
-
[25]
L H Hall, L B Kier. J. Chem. Inf. Comput. Sci., 35(6):1039~1045.
-
[26]
R Todeschini, V Consonni. Handbook of molecular descriptors. Germany Weinheim:Wiley-VCH Verlag, 2000.
-
[27]
T I Opera, C L Waller, G R Marshall. J. Med. Chem., 1994, 37(14):2206~2215.
-
[28]
M Ravi, A J Hopfinger, R E Hormann et al. J. Chem. Inf. Comput. Sci., 2001, 41(6):1587~1604.
-
[29]
O Mekenyan, S Dimitrov, P Schmieder et al. SAR QSAR Environ. Res., 2003, 14(5~6):361~371.
-
[30]
C Cortes, V Vapnik. Mach. Learn., 1995, 20(3):273~379.
-
[31]
A M Nassef, E T Sayed, H Rezk et al. Energ. Source. A, 2019, 41(17):2094~2103.
-
[32]
P D Wasserman. Neural Computing Theory and Practice. van Nostrand-Reinhold, New York, 1989.
-
[33]
T Kohonen. Neural Networks, 1988, 1(1):3~16.
-
[34]
L Rosa, M Camacho, A T Eliazar et al. Mol. Divers., 2018, 22(2):269~280.
-
[35]
S Wold, M Sjostrom. L Eriksson. Chemometr. Intell. Lab., 2001, 58(2):109~130.
- [36]
-
[37]
R Todeschini, P Gramatica. Quant. Struct-Act. Rel., 1997, 16(2):113~119.
-
[38]
R Todeschini, P Gramatica. Quant. Struct-Act Rel., 1997, 16(2):120~125.
-
[39]
V Consonni, R Todeschini, M Pavan et al. J. Chem. Inf. Comput. Sci., 2002, 42(3):682~692.
-
[40]
V Consonni, R Todeschini, M Pavan et al. J. Chem. Inf. Comput. Sci., 2002, 42:693~705.
-
[41]
B Mohar. Stud. Phys. Theor. Chem., 1989, 63:1~8.
-
[42]
A Voelkel. Comput. Chem., 1994, 18:1~4.
- [43]
-
[44]
M J Petitjean. J. Chem. Inf. Comput. Sci., 1992, 32:331~337.
-
[45]
J Devillers, A T Balaban. Topological Indices and Related Descriptors in QSAR and Drug Design. Gordon & Breach, Amsterdam (The Netherlands), 2000.
-
[46]
L B Kier, L H Hall. Molecular Connectivity in Structure-Activity Analysis. RSP-Wiley, Chichetser (UK), 1986.
- [47]
-
[48]
D Jiang, J G Zhou, N Li et al. Asian J. Ecotoxicol., 2014, 9(1):71~80.
- [49]
-
[50]
S Markovic, I Gutman, Z Bancevic. J. Serb. Chem. Soc., 1995, 60:33~636.
- [51]
-
[1]
-

-
-
[1]
Qianlang Wang , Jijun Sun , Qian Chen , Quanqin Zhao , Baojuan Xi . The Appeal of Organophosphorus Compounds: Clearing Their Name. University Chemistry, 2025, 40(4): 299-306. doi: 10.12461/PKU.DXHX202405205
-
[2]
Fengxiao Wang , Zhiwei Miao , Yaofeng Yuan . 有机磷化学与化学教学. University Chemistry, 2025, 40(8): 158-168. doi: 10.12461/PKU.DXHX202410077
-
[3]
Yongjian Zhang , Fangling Gao , Hong Yan , Keyin Ye . Electrochemical Transformation of Organosulfur Compounds. University Chemistry, 2025, 40(5): 311-317. doi: 10.12461/PKU.DXHX202407035
-
[4]
Yerong Chen , Bingbin Yang , Xinglei He , Yuqi Lin , Keyin Ye . Enzyme-Directed Evolution Enables Bioconversion of Organosilicon Compounds. University Chemistry, 2025, 40(10): 121-129. doi: 10.12461/PKU.DXHX202411054
-
[5]
Shuai Yuan , Yaofeng Yuan . Academician Chengye Yuan and Organic Phosphorus Chemistry. University Chemistry, 2025, 40(7): 393-400. doi: 10.12461/PKU.DXHX202409123
-
[6]
Yijing GU , Huan PANG , Rongmei ZHU . Applications of nickel-based metal-organic framework compounds in supercapacitors. Chinese Journal of Inorganic Chemistry, 2025, 41(10): 2029-2038. doi: 10.11862/CJIC.20250186
-
[7]
Jingjing QING , Fan HE , Zhihui LIU , Shuaipeng HOU , Ya LIU , Yifan JIANG , Mengting TAN , Lifang HE , Fuxing ZHANG , Xiaoming ZHU . Synthesis, structure, and anticancer activity of two complexes of dimethylglyoxime organotin. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1301-1308. doi: 10.11862/CJIC.20240003
-
[8]
Yan Kong , Wei Wei , Lekai Xu , Chen Chen . Electrochemical Synthesis of Organonitrogen Compounds from N-integrated CO2 Reduction Reaction. Acta Physico-Chimica Sinica, 2024, 40(8): 2307049-0. doi: 10.3866/PKU.WHXB202307049
-
[9]
Tianyun Chen , Ruilin Xiao , Xinsheng Gu , Yunyi Shao , Qiujun Lu . Synthesis, Crystal Structure, and Mechanoluminescence Properties of Lanthanide-Based Organometallic Complexes. University Chemistry, 2024, 39(5): 363-370. doi: 10.3866/PKU.DXHX202312017
-
[10]
Xiaodong Chen , Yumin Zhang . An Improved Simulated Annealing Algorithm for Predicting the Molecular Formulas of Organic Compounds. University Chemistry, 2025, 40(9): 19-24. doi: 10.12461/PKU.DXHX202408095
-
[11]
Junyuan Zhang , Zhiwei Miao . 有机磷杀虫剂的前世今生. University Chemistry, 2025, 40(6): 129-138. doi: 10.12461/PKU.DXHX202408118
-
[12]
Shicheng Yan . Experimental Teaching Design for the Integration of Scientific Research and Teaching: A Case Study on Organic Electrooxidation. University Chemistry, 2024, 39(11): 350-358. doi: 10.12461/PKU.DXHX202408036
-
[13]
Jiashuang Lu , Xiaoyang Xu , Youqing He , Mingyue Wu , Ruixin Shi , Wenfang Yu , Hang Lu , Ji Liu , Qingzeng Zhu . 生命健康中的有机硅高分子. University Chemistry, 2025, 40(8): 169-180. doi: 10.12461/PKU.DXHX202409143
-
[14]
Xiaogang YANG , Xinya ZHANG , Jing LI , Huilin WANG , Min LI , Xiaotian WEI , Xinci WU , Lufang MA . Synthesis, structure, and photoelectric properties of Zinc(Ⅱ)-triphenylamine based metal-organic framework. Chinese Journal of Inorganic Chemistry, 2025, 41(10): 2078-2086. doi: 10.11862/CJIC.20250167
-
[15]
Yuanqing Wang , Yusong Pan , Hongwu Zhu , Yanlei Xiang , Rong Han , Run Huang , Chao Du , Chengling Pan . Enhanced Catalytic Activity of Bi2WO6 for Organic Pollutants Degradation under the Synergism between Advanced Oxidative Processes and Visible Light Irradiation. Acta Physico-Chimica Sinica, 2024, 40(4): 2304050-0. doi: 10.3866/PKU.WHXB202304050
-
[16]
Qilong Fang , Yiqi Li , Jiangyihui Sheng , Quan Yuan , Jie Tan . Magical Pesticide Residue Detection Test Strips: Aptamer-based Lateral Flow Test Strips for Organophosphorus Pesticide Detection. University Chemistry, 2024, 39(5): 80-89. doi: 10.3866/PKU.DXHX202310004
-
[17]
Liang TANG , Jingfei NI , Kang XIAO , Xiangmei LIU . Synthesis and X-ray imaging application of lanthanide-organic complex-based scintillators. Chinese Journal of Inorganic Chemistry, 2024, 40(10): 1892-1902. doi: 10.11862/CJIC.20240139
-
[18]
Bao Jia , Yunzhe Ke , Shiyue Sun , Dongxue Yu , Ying Liu , Shuaishuai Ding . Innovative Experimental Teaching for the Preparation and Modification of Conductive Organic Polymer Thin Films in Undergraduate Courses. University Chemistry, 2024, 39(10): 271-282. doi: 10.12461/PKU.DXHX202404121
-
[19]
.
CCS Chemistry 综述推荐│绿色氧化新思路:光/电催化助力有机物高效升级
. CCS Chemistry, 2025, 7(10.31635/ccschem.024.202405369): -. -
[20]
Dan Liu . 可见光-有机小分子协同催化的不对称自由基反应研究进展. University Chemistry, 2025, 40(6): 118-128. doi: 10.12461/PKU.DXHX202408101
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(2272)
- HTML views(306)

 Login In
Login In




 DownLoad:
DownLoad: