Citation:
Li Shangfeng, Ma Xiuguang. Research Progress in the Synthesis of Homocamptothecin[J]. Chemistry,
;2018, 81(3): 203-208.

-
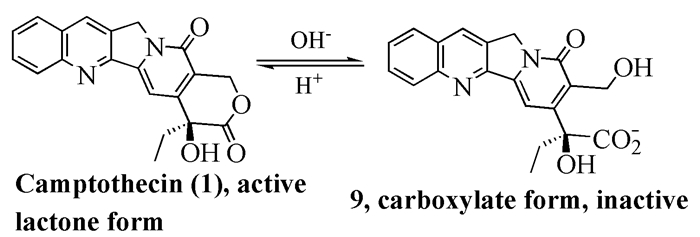
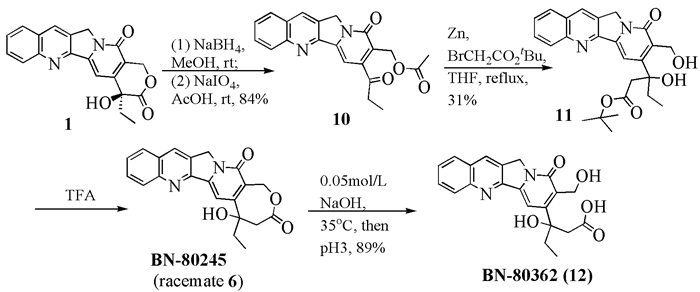
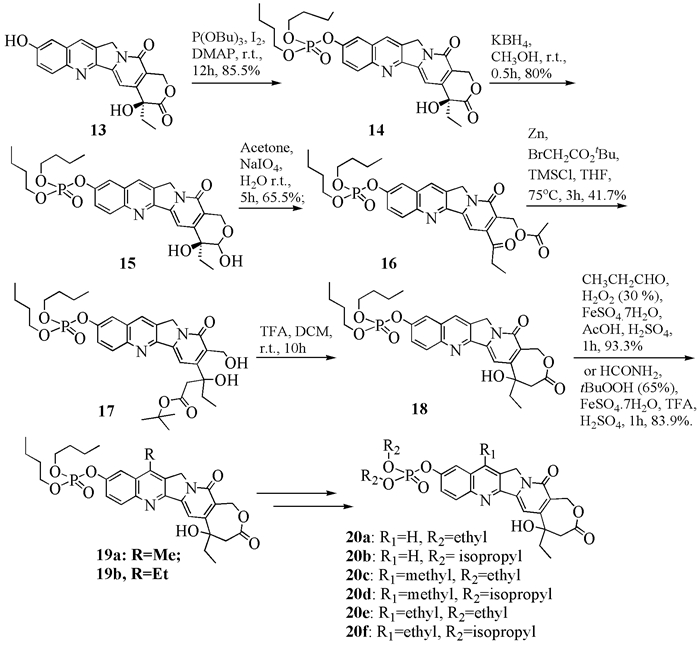
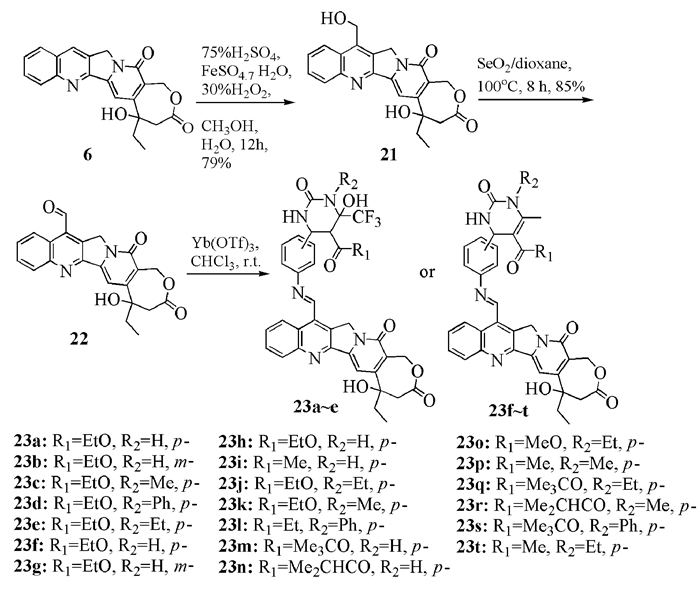
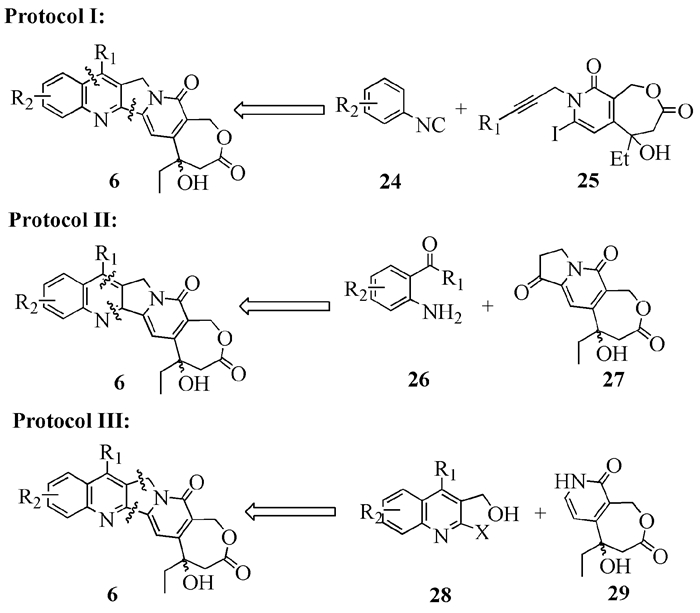
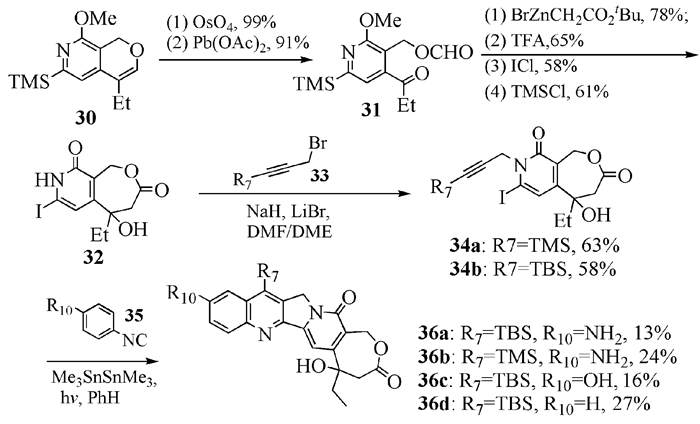
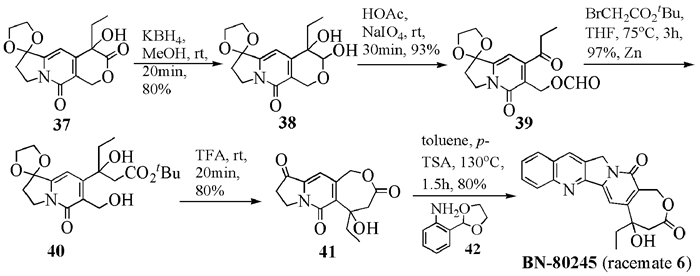
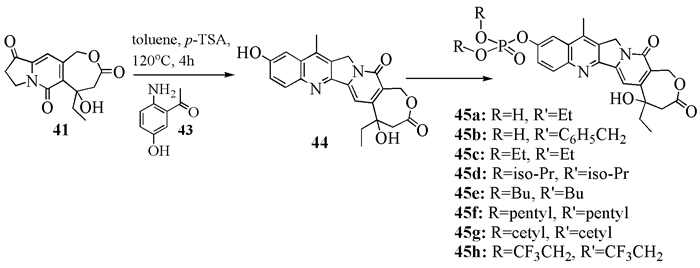
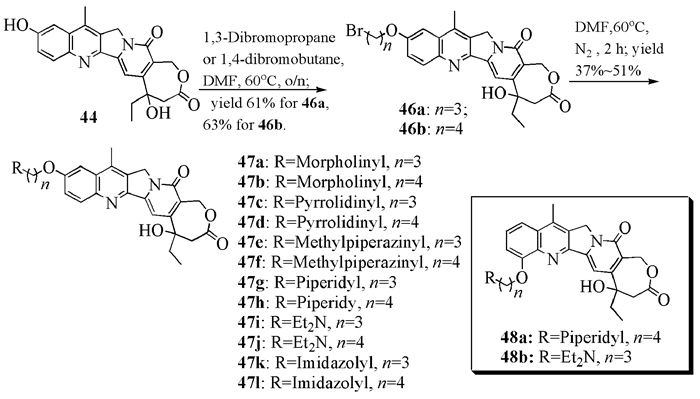
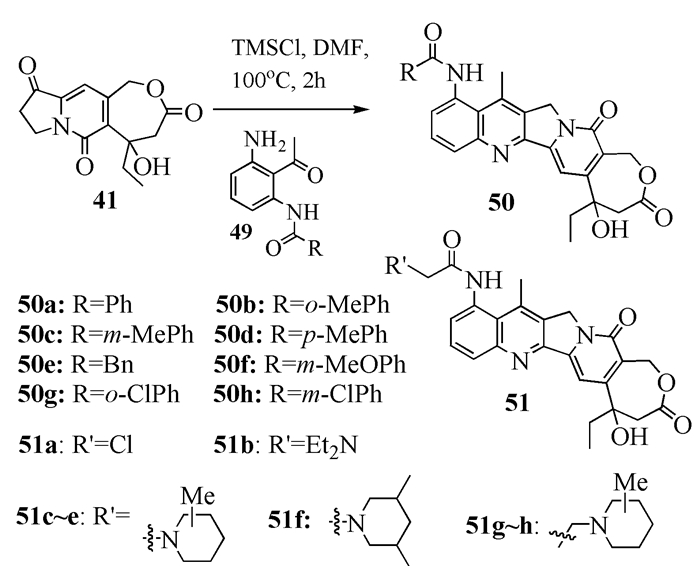
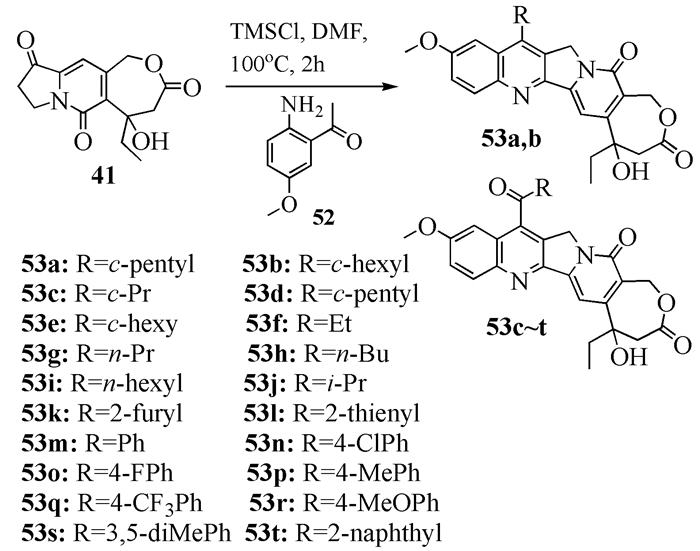
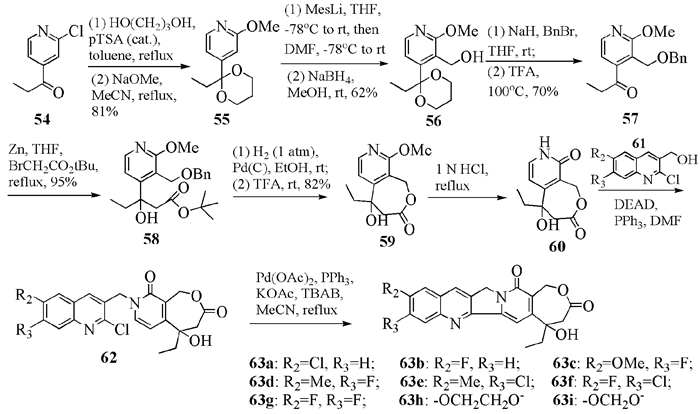
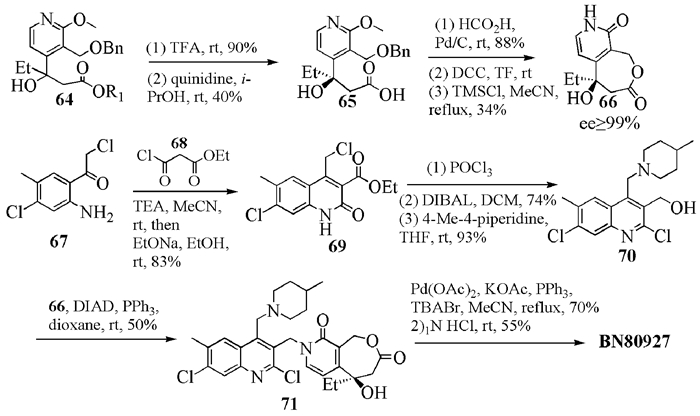
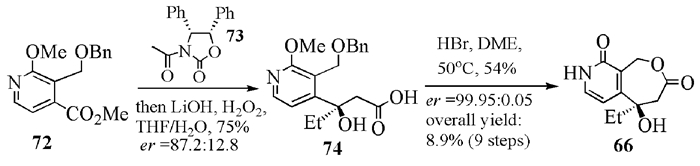
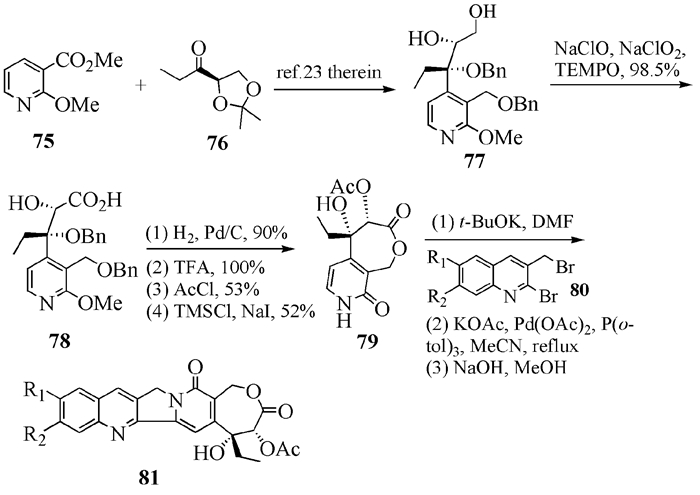
To date, there have been two ways to synthesize homocamptothecin including semi-synthesis and total synthesis. The former is simple and concise, yet with few substituent groups. It only produces the racemic homocamptothecin. On the contrary, the latter can introduce various substituent groups on ring A and B. This protocol can bring the asymmetric synthesis into practice, though the route seems a little lengthy. All the study advance of homocamptothecin at home and abroad was reviewed. Each group's synthetic protocol was introduced in depth along with their research results. The outlook of homocamptothecin in the field of medicine was foreshown, with the indication of the future research direction.
-
Keywords:
- Homocamptothecin,
- Topoisomerase I
-

-
- [1]
-
[2]
M E Wall, M C Wani, C E Cook et al. J. Am. Chem. Soc., 1966, 88:3888~3889.
-
[3]
K Mross, H Richly, N Schleucher et al. Ann. Oncol., 2004, 15:1284~1294.
-
[4]
O Lavergne, L L-Ginot, F P Rodas et al. J. Med. Chem., 1998, 41:5410~5419.
-
[5]
X Wu, X Sun, Z Guo et al. J. Am. Chem. Soc. 2014, 136:3579~3588.
- [6]
-
[7]
-
[8]
Y Q Zhang, H J Zhang, J Zhang et al. Aust. J. Chem., 2011, 64:1547~1553.
-
[9]
H F Hausheer, H Kochat, P Seetharamulu et al. WO: 9835940A1, 1998.
-
[10]
A E Gabarda, W Du, T Isarno et al. Tetrahedron, 2002, 58:6329~6341.
-
[11]
O Lavergne, L L Ginot, F P Rodas et al. Bioorg. Med. Chem. Lett., 1997, 7:2235~2238.
-
[12]
C Bailly, A Lansiaux, L Dassonneville et al. Biochemistry, 1999, 38:15556~15563.
-
[13]
D Demarquay, M Huchet, H Coulomb et al. Bioorg. Med. Chem. Lett., 1999, 9:2599~2602.
-
[14]
D Demarquay, M Huchet, H Coulomb. Anti Cancer Drugs, 2001, 12:9~19.
-
[15]
Z Mi, T G Burke. Biochemistry, 1994, 34:10325~10336.
-
[16]
-
[17]
L Zhu, P Cheng, N Lei et al. Arch. Pharm. Chem. Life Sci. 2011, 344:726~734.
-
[18]
-
[19]
D Z Li, C Y Wang, R H Liu et al. J. Asian Nat. Prod. Res., 2013, 15(11):1179~1188.
-
[20]
D Bom, D P Curran, A J Chavan et al. J. Med. Chem., 1999, 42:3018~3022.
-
[21]
D P Curran, W Du. Org. Lett., 2012, 4:3215~3218.
-
[22]
M C Wani, P E Ronman, J T Lindley et al. J. Med. Chem. 1980, 23:554~560.
-
[23]
Z Miao, C Sheng, W Zhang et al. Bioorg. Med. Chem., 2008, 16:1493~1510.
-
[24]
-
[25]
-
[26]
Z Miao, J Zhang, L You et al. Bioorg. Med. Chem., 2010, 18:3140~3146.
-
[27]
W Guo, W Liu, L Zhu et al. Chem. Biodiversity, 2011, 8(8):1539~1549.
-
[28]
W Liu, L Zhu, W Guo et al. Eur. J. Med. Chem., 2011, 46:2408~2414.
-
[29]
D Bigg, O Lavergne, F P Rodas et al. USP: 6339091 B1, 2002.
-
[30]
O Lavergne, D Demarquay, C Bailly et al. J. Med. Chem., 2000, 43:2285~2289.
-
[31]
R Peters, M Althaus, C Diolez et al. J. Org. Chem., 2006, 71:7583~7595.
-
[32]
Y Luo, S Yu, L Tong et al. Eur. J. Med. Chem., 2012, 54:281~286.
-

-
-
[1]
Zimo Shen , Tongwei Zhang , Zhiyi Zhu , Zonghao Gong , Qing Feng , Jinyi Yang , Zhen Li , Min Liu , Wei Qi . From Alkaloid to Anticancer Agent: The Transformative Journey of Camptothecin. University Chemistry, 2025, 40(10): 161-165. doi: 10.12461/PKU.DXHX202411027
-
[2]
Zhilian Liu , Wengui Wang , Hongxiao Yang , Yu Cui , Shoufeng Wang . Ideological and Political Education Design for the Synthesis of Irinotecan Drug Intermediate 7-Ethyl Camptothecin. University Chemistry, 2024, 39(2): 89-93. doi: 10.3866/PKU.DXHX202306012
-
[3]
Yihui Song , Shangshang Qin , Kai Wu , Chengyun Jin , Bin Yu . 生物化学在高水平创新型药学人才培养中的交叉融合应用——以去甲基化酶LSD1抑制剂的活性评价为例. University Chemistry, 2025, 40(6): 341-352. doi: 10.12461/PKU.DXHX202406018
-
[4]
Quanliang Chen , Zhaohui Zhou . Research on the Active Site of Nitrogenase over Fifty Years. University Chemistry, 2024, 39(7): 287-293. doi: 10.3866/PKU.DXHX202310133
-
[5]
Ya-Wen Zhang , Ming-Ming Gan , Li-Ying Sun , Ying-Feng Han . Supramolecular dinuclear silver(I) and gold(I) tetracarbene metallacycles and fluorescence sensing of penicillamine. Chinese Journal of Structural Chemistry, 2024, 43(9): 100356-100356. doi: 10.1016/j.cjsc.2024.100356
-
[6]
Bo YANG , Gongxuan LÜ , Jiantai MA . Corrosion inhibition of nickel-cobalt-phosphide in water by coating TiO2 layer. Chinese Journal of Inorganic Chemistry, 2025, 41(2): 365-384. doi: 10.11862/CJIC.20240063
-
[7]
Heng Zhang . Determination of All Rate Constants in the Enzyme Catalyzed Reactions Based on Michaelis-Menten Mechanism. University Chemistry, 2024, 39(4): 395-400. doi: 10.3866/PKU.DXHX202310047
-
[8]
Yerong Chen , Bingbin Yang , Xinglei He , Yuqi Lin , Keyin Ye . Enzyme-Directed Evolution Enables Bioconversion of Organosilicon Compounds. University Chemistry, 2025, 40(10): 121-129. doi: 10.12461/PKU.DXHX202411054
-
[9]
Xiaotian ZHU , Fangding HUANG , Wenchang ZHU , Jianqing ZHAO . Layered oxide cathode for sodium-ion batteries: Surface and interface modification and suppressed gas generation effect. Chinese Journal of Inorganic Chemistry, 2025, 41(2): 254-266. doi: 10.11862/CJIC.20240260
-
[10]
Zilin Hu , Yaoshen Niu , Xiaohui Rong , Yongsheng Hu . Suppression of Voltage Decay through Ni3+ Barrier in Anionic-Redox Active Cathode for Na-Ion Batteries. Acta Physico-Chimica Sinica, 2024, 40(6): 2306005-0. doi: 10.3866/PKU.WHXB202306005
-
[11]
Kai PENG , Xinyi ZHAO , Zixi CHEN , Xuhai ZHANG , Yuqiao ZENG , Jianqing JIANG . Progress in the application of high-entropy alloys and high-entropy ceramics in water electrolysis. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1257-1275. doi: 10.11862/CJIC.20240454
-
[12]
Xiaoyong ZHAI , Yao KOU , Pingru SU , Yu TANG . Lanthanide metal-organic framework with msw topology: Synthesis and the application in 2, 4, 6-trinitrophenol detection. Chinese Journal of Inorganic Chemistry, 2025, 41(10): 2087-2094. doi: 10.11862/CJIC.20250182
-
[13]
Chunmei GUO , Weihan YIN , Jingyi SHI , Jianhang ZHAO , Ying CHEN , Quli FAN . Facile construction and peroxidase-like activity of single-atom platinum nanozyme. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1633-1639. doi: 10.11862/CJIC.20240162
-
[14]
Yu Dai , Xueting Sun , Haoyu Wu , Naizhu Li , Guoe Cheng , Xiaojin Zhang , Fan Xia . Determination of the Michaelis Constant for Gold Nanozyme-Catalyzed Decomposition of Hydrogen Peroxide. University Chemistry, 2025, 40(5): 351-356. doi: 10.12461/PKU.DXHX202407052
-
[15]
Pengli GUAN , Renhu BAI , Xiuling SUN , Bin LIU . Trianiline-derived aggregation-induced emission luminogen probe for lipase detection and cell imaging. Chinese Journal of Inorganic Chemistry, 2025, 41(9): 1817-1826. doi: 10.11862/CJIC.20250058
-
[16]
Yue Sun , Liming Yang , Yaohang Cheng , Guanghui An , Guangming Li . Pd(I)-catalyzed ring-opening arylation of cyclopropyl-α-aminoamides: Access to α-ketoamide peptidomimetics. Chinese Chemical Letters, 2024, 35(6): 109250-. doi: 10.1016/j.cclet.2023.109250
-
[17]
Shuige Zhao , Pengcheng Yan , Peipei Liu , Haishan Liu , Ning Li , Peng Fu , Weiming Zhu . Pyridapeptides F‒I, cyclohexapeptides from marine sponge-derived Streptomyces sp. OUCMDZ-4539. Chinese Chemical Letters, 2024, 35(7): 108950-. doi: 10.1016/j.cclet.2023.108950
-
[18]
Jiaxuan Wang , Tonghe Liu , Bingxiang Wang , Ziwei Li , Yuzhong Niu , Hou Chen , Ying Zhang . Synthesis of polyhydroxyl-capped PAMAM dendrimer/silica composites for the adsorption of aqueous Hg(II) and Ag(I). Chinese Chemical Letters, 2024, 35(12): 109900-. doi: 10.1016/j.cclet.2024.109900
-
[19]
Xinyu ZENG , Guhua TANG , Jianming OUYANG . Inhibitory effect of Desmodium styracifolium polysaccharides with different content of carboxyl groups on the growth, aggregation and cell adhesion of calcium oxalate crystals. Chinese Journal of Inorganic Chemistry, 2024, 40(8): 1563-1576. doi: 10.11862/CJIC.20230374
-
[20]
Yajie Li , Bin Chen , Yiping Wang , Hui Xing , Wei Zhao , Geng Zhang , Siqi Shi . Inhibiting Dendrite Growth by Customizing Electrolyte or Separator to Achieve Anisotropic Lithium-Ion Transport: A Phase-Field Study. Acta Physico-Chimica Sinica, 2024, 40(3): 2305053-0. doi: 10.3866/PKU.WHXB202305053
-
[1]
Metrics
- PDF Downloads(16)
- Abstract views(1920)
- HTML views(739)

 Login In
Login In




 DownLoad:
DownLoad: