Citation:
Hu Daihua, Chen Wang. Advances in Syntheses and Biological Activities of Vitamin D2 Analogues[J]. Chemistry,
;2017, 80(8): 715-724.

-
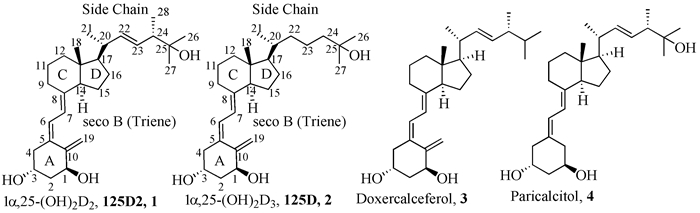
1α, 25-Dihydroxyvitamin D2(1α, 25-(OH)2-D2; 125D2) is the active metabolite form of vitamin D2, and 1α, 25-dihydroxyvitamin D3(1α, 25-(OH)2-D3; 125D) is the most active metabolite of vitamin D3. The biological activity of 125D2 and 125D is similar, but the therapeutic application of 125D is limited by induction of hypercalcemia. Vitamin D2 and analogues are thought to be generally less toxic than the respective vitamin D3 compounds. This article reviews the recent development of two 125D2 analogues (doxercalciferol and paricalcitol) showed good clinical effect in organic syntheses, and new research in the design and syntheses of the structural modifications in the side chain, 19-nor, C3-substituted in A ring, and nonadeuterated isotope labeling 125D2 analogues, and the relation between the structure and the biological activities, aimed at guiding significance to the syntheses and clinical development of new selective biological vitamin D2 analogues.
-

-
-
[1]
G Jones, H K Schnoes, H F De Luca. Biochemistry, 1975, 14(6):1250~1256.
-
[2]
H F DeLuca, L A Plum, M Clagett-Dame. J. Steroid. Biochem., 2007, 103(3):263~268.
-
[3]
N Urushino, K Yasuda, S Ikushiro et al. Biochem. Biophys. Res. Commun., 2009, 384(2):144~148.
-
[4]
G Jones, L A Baxter, H F De Luca et al. Biochemistry, 1976, 15(3):713~716.
-
[5]
-
[6]
M Chodyński, J Wietrzyk, E Marcinkowska et al. Steroids, 2002, 67(9):789~798.
- [7]
- [8]
-
[9]
B G Anderson, W E Bauta, Jr W R Cantrell. Org. Proc. Res. Dev., 2012, 16(5):967~975.
-
[10]
-
[11]
Y Ji, X Wang, R J Donnelly et al. J. Cell. Physiol., 2002, 191(2):198~207.
-
[12]
B Filip, M Milczarek, J Wietrzyk et al. J. Steroid. Biochem., 2010, 121(1):399~402.
-
[13]
S Nadkarni, M Chodynski, K Krajewski et al. J. Steroid. Biochem., 2016, 164:45~49.
-
[14]
A Piotrowska, J Wierzbicka, S Nadkarni et al. Int. J. Mol. Sci., 2016, 17(1):76.
-
[15]
M Chodyński, J Wietrzyk, E Marcinkowska et al. Steroids, 2002, 67(9):789~798.
-
[16]
J Wietrzyk, D Nevozhay, M Milczarek et al. Cancer. Chemoth. Pharm., 2008, 62(5):787~797.
-
[17]
H Baurska, A Klopot, M Kielbinski et al. J. Steroid. Biochem., 2011, 126(1):46~54.
-
[18]
N R Bolla, A Corcoran, K Yasuda et al. J. Steroid. Biochem., 2016, 164:50~55.
-
[19]
Z Gándara, M Pérez, X Pérez-García et al. Tetrahed. Lett., 2009, 50(34):4874~4877.
-
[20]
Z Gándara, M Pérez, D G Salomón et al. Bioorg. Med. Chem. Lett., 2012, 22(19):6276~6279.
-
[21]
S Vinhas, S Vázquez, J E Rodríguez-Borges et al. J. Steroid. Biochem., 2016
- [22]
- [23]
-
[24]
L Li, L Yue, J Xue et al. Chin. Sci. Bull., 2012, 57(14):1616~1619.
-
[25]
A Toyoda, H Nagai, T Yamada et al. Tetrahedron, 2009, 65(48):10002~10008.
-
[26]
R Samala, S Sharma, M K Basu et al. Tetrahed. Lett., 2016, 57(12):1309~1312.
-
[27]
A Pietraszek, M Malińska, M Chodyński et al. Steroids, 2013, 78(10):1003~1014.
-
[28]
M Nachliely, E Sharony, A Kutner et al. J. Steroid. Biochem., 2016, 164:59~65.
- [29]
-
[30]
R Sigüeiro, R Otero, P González-Berdullas et al. J. Steroid. Biochem., 2015, 148:31~33.
-
[31]
-
[32]
R Bouillon, W H Okamura, A W Norman. Endocr. Rev., 1995, 16(2):200~257.
-
[33]
R Sigüeiro, A Álvarez, R Otero et al. J. Steroid. Biochem., 2014, 144:204~206.
-
[1]
-

-
-
[1]
Xin MA , Ya SUN , Na SUN , Qian KANG , Jiajia ZHANG , Ruitao ZHU , Xiaoli GAO . A Tb2 complex based on polydentate Schiff base: Crystal structure, fluorescence properties, and biological activity. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1347-1356. doi: 10.11862/CJIC.20230357
-
[2]
Lifang HE , Wenjie TANG , Yaoze LUO , Mingsheng LIANG , Jianxin TANG , Yuxuan WU , Fuxing ZHANG , Xiaoming ZHU . Synthesis, structure, and anticancer activity of two dialkyltin complexes constructed based on 2, 2′-bipyridin-6, 6′-dicarboxylic acid. Chinese Journal of Inorganic Chemistry, 2025, 41(8): 1601-1609. doi: 10.11862/CJIC.20250012
-
[3]
Jing WU , Puzhen HUI , Huilin ZHENG , Pingchuan YUAN , Chunfei WANG , Hui WANG , Xiaoxia GU . Synthesis, crystal structures, and antitumor activities of transition metal complexes incorporating a naphthol-aldehyde Schiff base ligand. Chinese Journal of Inorganic Chemistry, 2024, 40(12): 2422-2428. doi: 10.11862/CJIC.20240278
-
[4]
Xinting XIONG , Zhiqiang XIONG , Panlei XIAO , Xuliang NIE , Xiuying SONG , Xiuguang YI . Synthesis, crystal structures, Hirshfeld surface analysis, and antifungal activity of two complexes Na(Ⅰ)/Cd(Ⅱ) assembled by 5-bromo-2-hydroxybenzoic acid ligands. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1661-1670. doi: 10.11862/CJIC.20240145
-
[5]
Haitang WANG , Yanni LING , Xiaqing MA , Yuxin CHEN , Rui ZHANG , Keyi WANG , Ying ZHANG , Wenmin WANG . Construction, crystal structures, and biological activities of two LnⅢ3 complexes. Chinese Journal of Inorganic Chemistry, 2024, 40(8): 1474-1482. doi: 10.11862/CJIC.20240188
-
[6]
Bin SUN , Heyan JIANG . Glucose-modified bis-Schiff bases: Synthesis and bio-activities in Alzheimer′s disease therapy. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1338-1350. doi: 10.11862/CJIC.20240428
-
[7]
Lixing ZHANG , Yaowen WANG , Xu HAN , Junhong ZHOU , Jinghui WANG , Liping LI , Guangshe LI . Research progress in the synthesis of fluorine-containing perovskites and their derivatives. Chinese Journal of Inorganic Chemistry, 2025, 41(9): 1689-1701. doi: 10.11862/CJIC.20250007
-
[8]
Jiaming Xu , Yu Xiang , Weisheng Lin , Zhiwei Miao . Research Progress in the Synthesis of Cyclic Organic Compounds Using Bimetallic Relay Catalytic Strategies. University Chemistry, 2024, 39(3): 239-257. doi: 10.3866/PKU.DXHX202309093
-
[9]
Siran Wang , Yinuo Wang , Yilong Zhao , Dazhen Xu . Advances in the Application and Preparation of Rhodanine and Its Derivatives. University Chemistry, 2025, 40(5): 318-327. doi: 10.12461/PKU.DXHX202407033
-
[10]
Chunling Qin , Shuang Chen , Hassanien Gomaa , Mohamed A. Shenashen , Sherif A. El-Safty , Qian Liu , Cuihua An , Xijun Liu , Qibo Deng , Ning Hu . Regulating HER and OER Performances of 2D Materials by the External Physical Fields. Acta Physico-Chimica Sinica, 2024, 40(9): 2307059-0. doi: 10.3866/PKU.WHXB202307059
-
[11]
Ying Chen , Ronghua Yan , Weiyan Yin . Research Progress on the Synthesis of Metal Single-Atom Catalysts and Their Applications in Electrocatalytic Hydrogen Evolution Reactions. University Chemistry, 2025, 40(9): 344-353. doi: 10.12461/PKU.DXHX202503066
-
[12]
Xinyi Zhang , Kai Ren , Yanning Liu , Zhenyi Gu , Zhixiong Huang , Shuohang Zheng , Xiaotong Wang , Jinzhi Guo , Igor V. Zatovsky , Junming Cao , Xinglong Wu . Progress on Entropy Production Engineering for Electrochemical Catalysis. Acta Physico-Chimica Sinica, 2024, 40(7): 2307057-0. doi: 10.3866/PKU.WHXB202307057
-
[13]
Lei Feng , Ze-Min Zhu , Ying Yang , Zongbin He , Jiafeng Zou , Man-Bo Li , Yan Zhao , Zhikun Wu . Long-Pursued Structure of Au23(S-Adm)16 and the Unexpected Doping Effects. Acta Physico-Chimica Sinica, 2024, 40(5): 2305029-0. doi: 10.3866/PKU.WHXB202305029
-
[14]
Liping GUO . Synthesis and crystal structure characterization of yttrium imido complex: The reactivity of 2-substituted-1-amino-o-carborane with yttrium dialkyl complex. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1409-1415. doi: 10.11862/CJIC.20250065
-
[15]
Yuanyuan Ping , Wangqing Kong . 光催化碳氢键官能团化合成1-苯基-1,2-乙二醇. University Chemistry, 2025, 40(6): 238-247. doi: 10.12461/PKU.DXHX202408092
-
[16]
Yajin Li , Huimin Liu , Lan Ma , Jiaxiong Liu , Dehua He . Photothermal Synthesis of Glycerol Carbonate via Glycerol Carbonylation with CO2 over Au/Co3O4-ZnO Catalyst. Acta Physico-Chimica Sinica, 2024, 40(9): 2308005-0. doi: 10.3866/PKU.WHXB202308005
-
[17]
Yifeng TAN , Ping CAO , Kai MA , Jingtong LI , Yuheng WANG . Synthesis of pentaerythritol tetra(2-ethylthylhexoate) catalyzed by h-MoO3/SiO2. Chinese Journal of Inorganic Chemistry, 2024, 40(11): 2155-2162. doi: 10.11862/CJIC.20240147
-
[18]
Jingjing QING , Fan HE , Zhihui LIU , Shuaipeng HOU , Ya LIU , Yifan JIANG , Mengting TAN , Lifang HE , Fuxing ZHANG , Xiaoming ZHU . Synthesis, structure, and anticancer activity of two complexes of dimethylglyoxime organotin. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1301-1308. doi: 10.11862/CJIC.20240003
-
[19]
Linjie ZHU , Xufeng LIU . Synthesis, characterization and electrocatalytic hydrogen evolution of two di-iron complexes containing a phosphine ligand with a pendant amine. Chinese Journal of Inorganic Chemistry, 2025, 41(5): 939-947. doi: 10.11862/CJIC.20240416
-
[20]
Maitri Bhattacharjee , Rekha Boruah Smriti , R. N. Dutta Purkayastha , Waldemar Maniukiewicz , Shubhamoy Chowdhury , Debasish Maiti , Tamanna Akhtar . Synthesis, structural characterization, bio-activity, and density functional theory calculation on Cu(Ⅱ) complexes with hydrazone-based Schiff base ligands. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1409-1422. doi: 10.11862/CJIC.20240007
-
[1]
Metrics
- PDF Downloads(16)
- Abstract views(2913)
- HTML views(314)

 Login In
Login In




 DownLoad:
DownLoad: