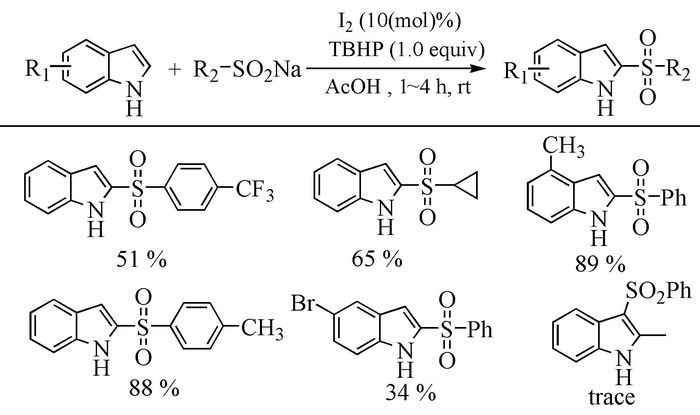
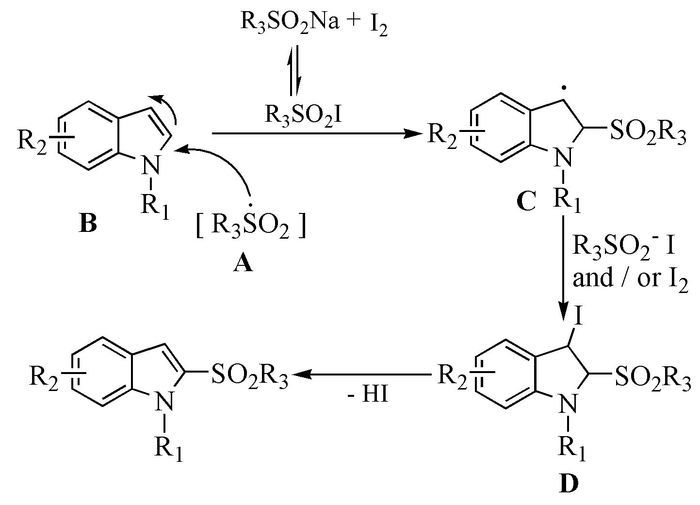
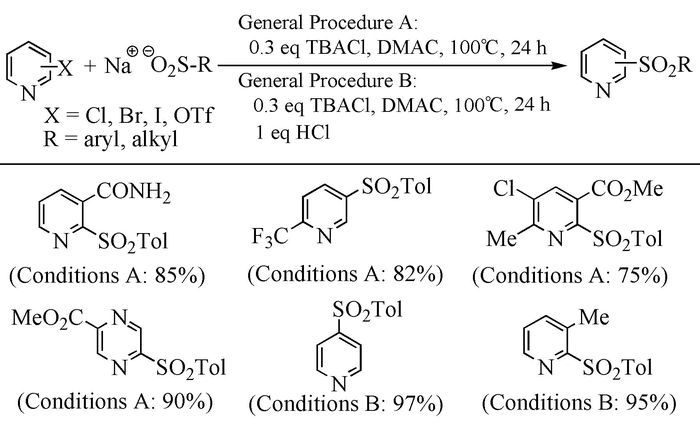
Citation:
Xiao Caiqin. Progress in the Syntheses of Sulfones[J]. Chemistry,
;2017, 80(10): 908-917.

-
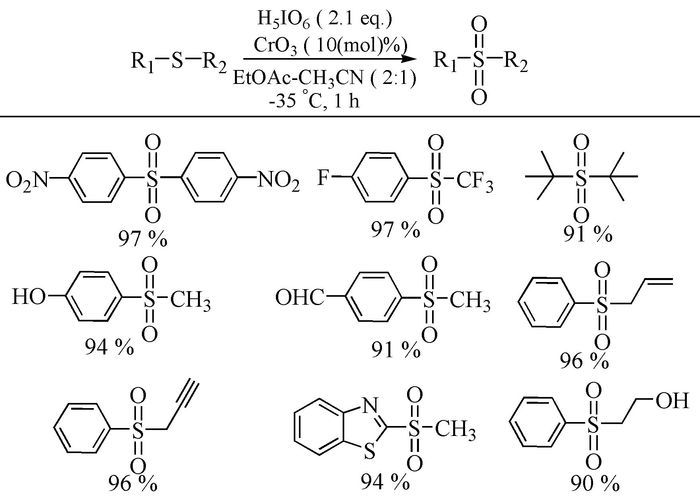
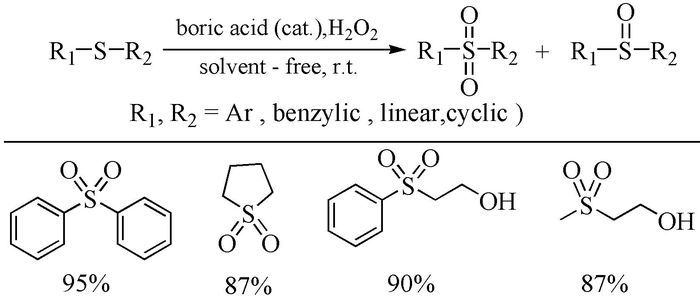
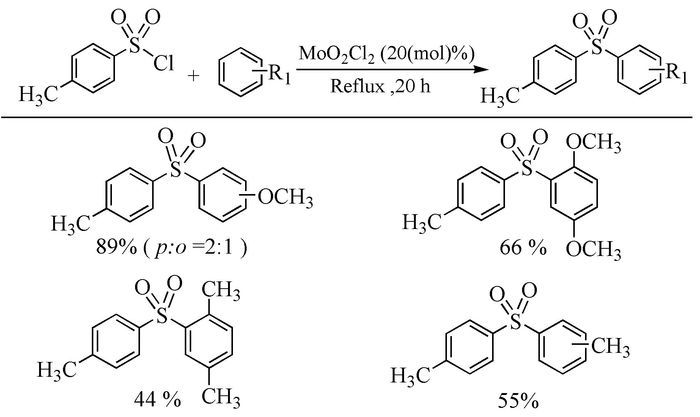
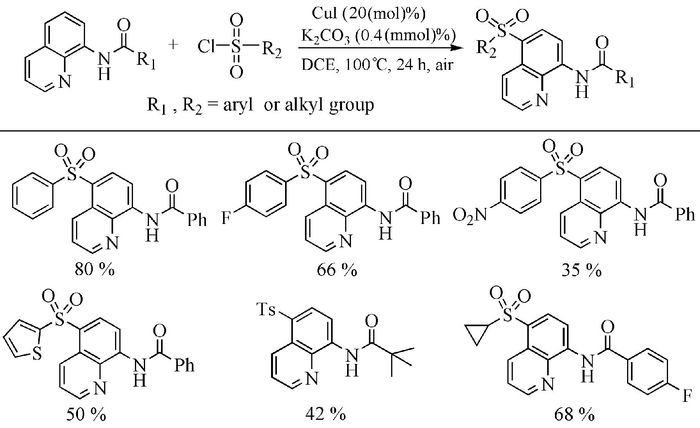
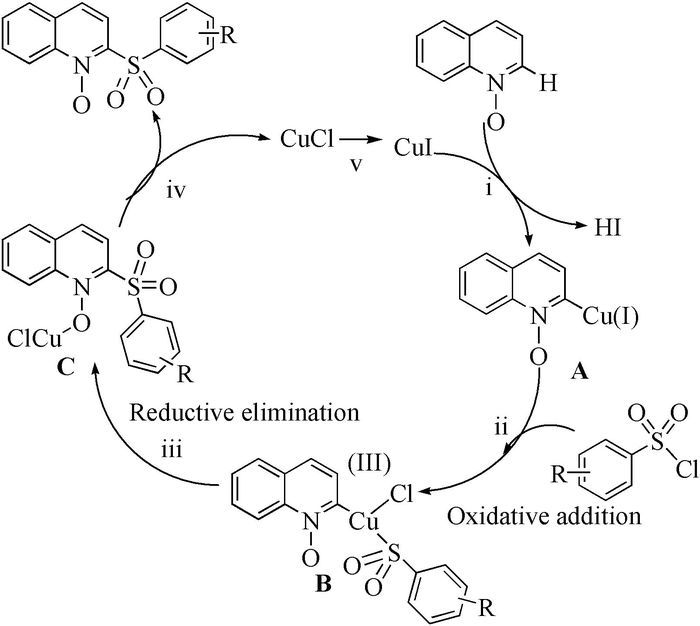
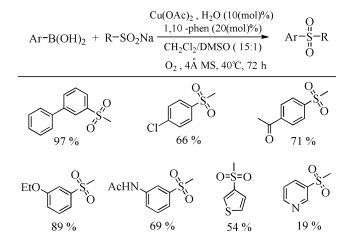
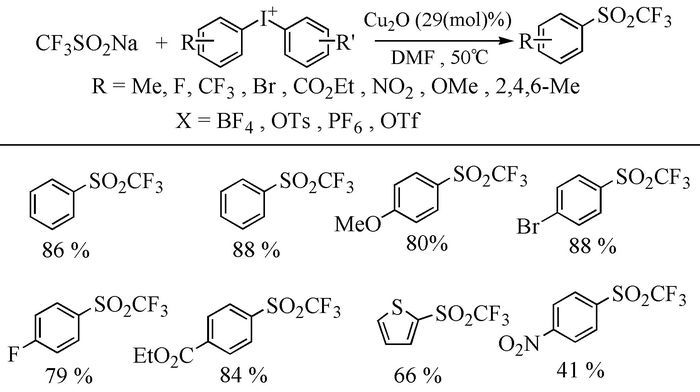
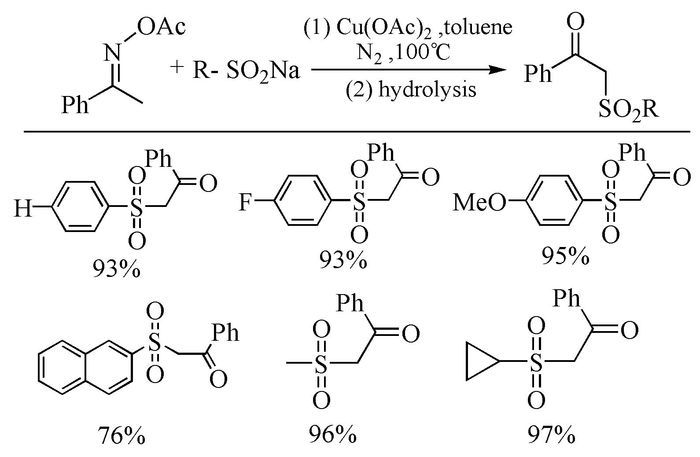
The broad spectrum of biological activity of sulfone compounds endows a wide applications in chemistry, medicine, agrochemistry and material science.Being an strong electron-withdrawing group, elongation of carbon chain could easily achieved via reaction of aliphatic sulfones with various electrophiles under basic condition.As a result, novel synthetic methodologies have continuous been developed especially during the past decades.This paper aimed at summarizing the synthetic methods of the sulfone compounds appeared in recent years, emphasizing the oxidation of sulfides, sulfonylation with sulfonyl chlorides and coupling of sulfinic acid salts.
-

-
-
[1]
B J Zhang, A M Wassermann, M Vogt et al. J. Chem. Inf. Model., 2012, 52(12):3138~3143.
-
[2]
J S Scott, A M Birch, K J Brocklehurst et al. J. Med. Chem., 2012, 55:5361~5379.
-
[3]
W M Xu, F F Han, M He et al. J. Agric. Food Chem., 2012, 60:1036~1041.
- [4]
-
[5]
M Artico, R Silvestri, S Massa et al. J. Med. Chem., 1996, 39(2):522~530.
-
[6]
T M Williams, T M Ciccarone, S C Mac Tough et al. J. Med. Chem., 1993, 36(9):1291~1294.
-
[7]
W T Li, D R Hwang, J S Song et al. J. Med. Chem., 2010, 53:2409~2417.
-
[8]
R Silvestri, M Artico, G L Regina et al. Farmaco, 2004, 59:201~210.
-
[9]
A Basak, M Goswami, A Rajkumar et al. Bioorg. Med. Chem. Lett., 2015, 25(10):2225~2237.
-
[10]
S Consalvia, S Alfonsoa, A D Capuab et al. Bioorg. Med. Chem. Lett., 2015, 23(4):810~820.
-
[11]
L M Ni, X S Zheng, P K Somers et al. Bioorg. Med. Chem. Lett., 2003, 13(4):745~748.
-
[12]
D H Boschelli, J B Kramer, S S Khatana et al. J. Med. Chem., 1995, 38(22):4597~4614.
-
[13]
T K Pal, S Dey, T Pathak. J. Org. Chem., 2011, 76(9):3034~3041.
-
[14]
D Lee, C L Williamson, L N Chan et al. J. Am. Chem. Soc., 2012, 134:8260~8267.
- [15]
-
[16]
R Beckerbauer, B E Smart. J. Org. Chem., 1996, 60:6186~6187.
-
[17]
R S Varma, R K Saini, H M Meshram. Tetrahed. Lett., 1997, 38:6525~6528.
-
[18]
L Xu, J Cheng, M L Trudell. J. Org. Chem., 2003, 68:5388~5391.
- [19]
- [20]
-
[21]
M Rahimizadeh, G Rajabzadeh, S M Khatami et al. J. Mol. Catal. A, 2010, 323:59~64.
- [22]
- [23]
-
[24]
B Maleki, S Hemmati, A Sedrpoushan et al. RSC Adv., 2014, 4:40505~40510.
-
[25]
R D Chakravarthy, V Ramkumara, D K Chand. Green Chem., 2014, 16:2190~2196.
- [26]
-
[27]
C G Frost, J P Hartleya, D Griffinb. Tetrahed. Lett., 2002, 43:4789~4791
-
[28]
G A Olah, A Orlinkov, A B Oxyglou et al. J. Org. Chem., 1985, 50(8):1306~1309.
-
[29]
R P Singh, R M Kamble, K L Chandra et al. Tetrahed. Lett., 2001, 57:241~247.
-
[30]
G A Olah, T Mathew, G K S Parakash. Chem. Commun., 2001, 1696~1697.
-
[31]
S J Nara, J R Harjani, M F Salunkhe. J. Org. Chem., 2001, 66:8616~8620.
-
[32]
J Marquie, A Laporterie, J Dubac. J. Org. Chem., 2001, 66:421~425.
-
[33]
V Garzya, I T Forbes, S Lauru et al. Tetrahed. Lett., 2004, 45:1499~1501.
-
[34]
S Repichet, C Le Roux, P Hernandez et al. J. Org. Chem., 1999, 64(17):6479~6483.
-
[35]
D U Singh, P R Singh, S D Samant. Tetrahed. Lett., 2004, 45:9079~9082.
-
[36]
R G D Noronha, A C Fernandes, C C Romão. Tetrahed. Lett., 2009, 50:1407~1410.
-
[37]
H W Liang, K Jiang, W Ding et al. Chem. Commun., 2015, 51:16928~16931.
-
[38]
Z Y Wu, H Y Song, X L Cui et al. Org. Lett., 2013, 15(6):1270~1273.
-
[39]
Y Fu, Q S Xu, Q Z Li et al. Org. Biomol. Chem., 2017, 13:2841~2845.
-
[40]
-
[41]
S Cacchi, G Fabrizi, A Goggiamani et al. J. Org. Chem., 2004, 69:5608~5614.
- [42]
- [43]
-
[44]
C Beaulieu, D Guay, Z Y Wang et al. Tetrahed. Lett., 2004, 45:3233~3236.
- [45]
-
[46]
R Kuwano, Y Kondo, T Shirahama. Org. Lett., 2005, 7(14):2973~2975.
-
[47]
A Kar, I A Sayyed, W F Lo et al. Org. Lett., 2007, 9(17):3405~3408.
- [48]
-
[49]
M L Kantam, B Neelima, B Sreedhar et al. Synlett, 2008, 10:1455~1458.
-
[50]
X Y Zhou, J Y Luo, Liu J et al. Org. Lett., 2011, 13(6):1432~1435.
-
[51]
X S Wu, Y Chen, M B Li et al. J. Am. Chem. Soc., 2012, 134:14694~14698.
-
[52]
S C Cullen, S Shekhar, N K Nere. J. Org. Chem., 2013, 78:12194~12201.
-
[53]
X D Tang, L B Huang, Y L Xu et al. Angew. Chem. Int. Ed., 2014, 53:4205~4208.
- [54]
-
[55]
P Katrun, C Mueangkaew, M Pohmakotr et al. J. Org. Chem., 2014, 79(4):1778~1785.
-
[56]
F H Xiao, S Q Chen, Y Chen et al. Chem. Commun., 2015, 51:652~654.
-
[57]
J Chen, J C Mao, Y Zheng et al. Tetrahedron, 2015, 71(31):5059~5063.
-
[58]
V K Yadav, V P Srivastava, L D S Yadav. Tetrahed. Lett., 2016, 57(21):2236~2238.
-
[59]
K M Maloney, J T Kuethe, K Linn. Org. Lett., 2011, 13(1):102~105.
-
[60]
S Liang, R Y Zhang, L Y Xi et al. J. Org. Chem., 2013, 78:11874~11880.
- [61]
-
[62]
- [63]
-
[64]
Z J Liu, R C Larock. J. Am. Chem. Soc., 2005, 127(38):13112~13113.
-
[65]
U K Tambar, B M Stoltz. J. Am. Chem. Soc., 2005, 127(15):5340~5341.
-
[66]
T T Tayan, M Jeganmohan, M J Cheng. J. Am. Chem. Soc., 2006, 128(7):2232~2233.
-
[67]
J L Henderson, A S Edwards, M F Greaney. J. Am. Chem. Soc., 2006, 128(23):7426~7427.
- [68]
-
[1]
-

-
-
[1]
Jing WU , Puzhen HUI , Huilin ZHENG , Pingchuan YUAN , Chunfei WANG , Hui WANG , Xiaoxia GU . Synthesis, crystal structures, and antitumor activities of transition metal complexes incorporating a naphthol-aldehyde Schiff base ligand. Chinese Journal of Inorganic Chemistry, 2024, 40(12): 2422-2428. doi: 10.11862/CJIC.20240278
-
[2]
Xinting XIONG , Zhiqiang XIONG , Panlei XIAO , Xuliang NIE , Xiuying SONG , Xiuguang YI . Synthesis, crystal structures, Hirshfeld surface analysis, and antifungal activity of two complexes Na(Ⅰ)/Cd(Ⅱ) assembled by 5-bromo-2-hydroxybenzoic acid ligands. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1661-1670. doi: 10.11862/CJIC.20240145
-
[3]
Lifang HE , Wenjie TANG , Yaoze LUO , Mingsheng LIANG , Jianxin TANG , Yuxuan WU , Fuxing ZHANG , Xiaoming ZHU . Synthesis, structure, and anticancer activity of two dialkyltin complexes constructed based on 2, 2′-bipyridin-6, 6′-dicarboxylic acid. Chinese Journal of Inorganic Chemistry, 2025, 41(8): 1601-1609. doi: 10.11862/CJIC.20250012
-
[4]
Jiaming Xu , Yu Xiang , Weisheng Lin , Zhiwei Miao . Research Progress in the Synthesis of Cyclic Organic Compounds Using Bimetallic Relay Catalytic Strategies. University Chemistry, 2024, 39(3): 239-257. doi: 10.3866/PKU.DXHX202309093
-
[5]
Lixing ZHANG , Yaowen WANG , Xu HAN , Junhong ZHOU , Jinghui WANG , Liping LI , Guangshe LI . Research progress in the synthesis of fluorine-containing perovskites and their derivatives. Chinese Journal of Inorganic Chemistry, 2025, 41(9): 1689-1701. doi: 10.11862/CJIC.20250007
-
[6]
Ying Chen , Ronghua Yan , Weiyan Yin . Research Progress on the Synthesis of Metal Single-Atom Catalysts and Their Applications in Electrocatalytic Hydrogen Evolution Reactions. University Chemistry, 2025, 40(9): 344-353. doi: 10.12461/PKU.DXHX202503066
-
[7]
Bin SUN , Heyan JIANG . Glucose-modified bis-Schiff bases: Synthesis and bio-activities in Alzheimer′s disease therapy. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1338-1350. doi: 10.11862/CJIC.20240428
-
[8]
Lili Jiang , Shaoyu Zheng , Xuejiao Liu , Xiaomin Xie . Copper-Catalyzed Oxidative Coupling Reactions for the Synthesis of Aryl Sulfones: A Fundamental and Exploratory Experiment for Undergraduate Teaching. University Chemistry, 2025, 40(7): 267-276. doi: 10.12461/PKU.DXHX202408004
-
[9]
Xinyi Zhang , Kai Ren , Yanning Liu , Zhenyi Gu , Zhixiong Huang , Shuohang Zheng , Xiaotong Wang , Jinzhi Guo , Igor V. Zatovsky , Junming Cao , Xinglong Wu . Progress on Entropy Production Engineering for Electrochemical Catalysis. Acta Physico-Chimica Sinica, 2024, 40(7): 2307057-0. doi: 10.3866/PKU.WHXB202307057
-
[10]
Lei Feng , Ze-Min Zhu , Ying Yang , Zongbin He , Jiafeng Zou , Man-Bo Li , Yan Zhao , Zhikun Wu . Long-Pursued Structure of Au23(S-Adm)16 and the Unexpected Doping Effects. Acta Physico-Chimica Sinica, 2024, 40(5): 2305029-0. doi: 10.3866/PKU.WHXB202305029
-
[11]
Chunling Qin , Shuang Chen , Hassanien Gomaa , Mohamed A. Shenashen , Sherif A. El-Safty , Qian Liu , Cuihua An , Xijun Liu , Qibo Deng , Ning Hu . Regulating HER and OER Performances of 2D Materials by the External Physical Fields. Acta Physico-Chimica Sinica, 2024, 40(9): 2307059-0. doi: 10.3866/PKU.WHXB202307059
-
[12]
Geyang Song , Dong Xue , Gang Li . Recent Advances in Transition Metal-Catalyzed Synthesis of Anilines from Aryl Halides. University Chemistry, 2024, 39(2): 321-329. doi: 10.3866/PKU.DXHX202308030
-
[13]
Aidang Lu , Yunting Liu , Yanjun Jiang . Comprehensive Organic Chemistry Experiment: Synthesis and Characterization of Triazolopyrimidine Compounds. University Chemistry, 2024, 39(8): 241-246. doi: 10.3866/PKU.DXHX202401029
-
[14]
Chi Li , Jichao Wan , Qiyu Long , Hui Lv , Ying Xiong . N-Heterocyclic Carbene (NHC)-Catalyzed Amidation of Aldehydes with Nitroso Compounds. University Chemistry, 2024, 39(5): 388-395. doi: 10.3866/PKU.DXHX202312016
-
[15]
Yanhui Zhong , Ran Wang , Zian Lin . Analysis of Halogenated Quinone Compounds in Environmental Water by Dispersive Solid-Phase Extraction with Liquid Chromatography-Triple Quadrupole Mass Spectrometry. University Chemistry, 2024, 39(11): 296-303. doi: 10.12461/PKU.DXHX202402017
-
[16]
Feng Han , Fuxian Wan , Ying Li , Congcong Zhang , Yuanhong Zhang , Chengxia Miao . Comprehensive Organic Chemistry Experiment: Phosphotungstic Acid-Catalyzed Direct Conversion of Triphenylmethanol for the Synthesis of Oxime Ethers. University Chemistry, 2025, 40(3): 342-348. doi: 10.12461/PKU.DXHX202405181
-
[17]
Xilin Zhao , Xingyu Tu , Zongxuan Li , Rui Dong , Bo Jiang , Zhiwei Miao . Research Progress in Enantioselective Synthesis of Axial Chiral Compounds. University Chemistry, 2024, 39(11): 158-173. doi: 10.12461/PKU.DXHX202403106
-
[18]
Liping GUO . Synthesis and crystal structure characterization of yttrium imido complex: The reactivity of 2-substituted-1-amino-o-carborane with yttrium dialkyl complex. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1409-1415. doi: 10.11862/CJIC.20250065
-
[19]
Zhen Yao , Bing Lin , Youping Tian , Tao Li , Wenhui Zhang , Xiongwei Liu , Wude Yang . Visible-Light-Mediated One-Pot Synthesis of Secondary Amines and Mechanistic Exploration. University Chemistry, 2024, 39(5): 201-208. doi: 10.3866/PKU.DXHX202311033
-
[20]
Yan Kong , Wei Wei , Lekai Xu , Chen Chen . Electrochemical Synthesis of Organonitrogen Compounds from N-integrated CO2 Reduction Reaction. Acta Physico-Chimica Sinica, 2024, 40(8): 2307049-0. doi: 10.3866/PKU.WHXB202307049
-
[1]
Metrics
- PDF Downloads(247)
- Abstract views(10079)
- HTML views(4104)

 Login In
Login In





 DownLoad:
DownLoad: