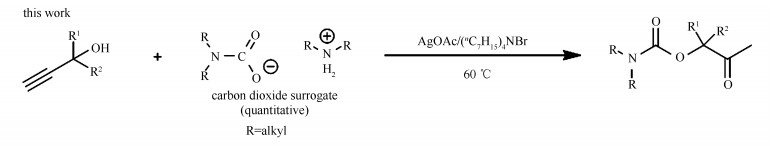
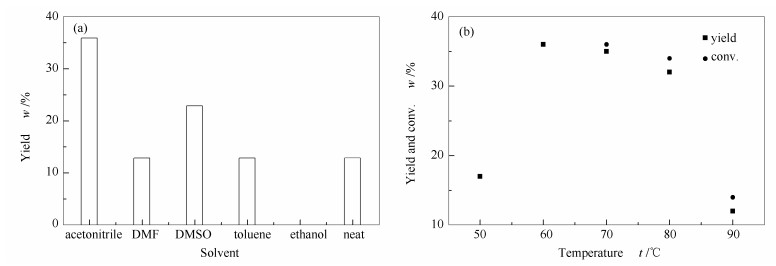
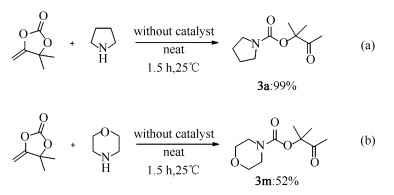
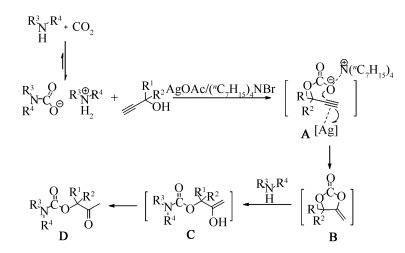
-
[1]
HE Liang-nian.CO2 Chemistry[M].Beijing:Science Press, 2013.
-
[2]
SAKAKURA T, CHOI J C, YASUDA H. Transformation of carbon dioxide[J]. Chem Rev,
2007,107(6):2365-2387.
doi: 10.1021/cr068357u
-
[3]
SONG Q W, ZHOU Z H, HE L N. Efficient, selective and sustainable catalysis of carbon dioxide[J]. Green Chem,
2017,19(16):3707-3728.
doi: 10.1039/C7GC00199A
-
[4]
GAO Jian, MIAO Cheng-xia, WANG Jing-lun, HE Liang-nian. Recent advances in utilization of carbon dioxide as a renewable resource[J]. Petrochem Technol,
2010,39(5):465-475.
-
[5]
HE M Y, SUN Y H, HAN B X. Green carbon science:Scientific basis for integrating carbon resource processing, utilization, and recycling[J]. Angew Chem Int Ed,
2013,52(37):9620-9633.
doi: 10.1002/anie.201209384
-
[6]
LIU An-hua, HE Liang-nian, GAO Jian, YANG Zhen-zhen, LI Yu-nong, LI Bin, YU Bing. Carbon dioxide chemistry:Catalytic conversion of CO2 into value-added fuels and chemicals[J]. Chin J Synth Chem,
2010,18(S1):80-91.
-
[7]
ARESTA M, DIBENEDETTO A, FRACCHIOLLA E, GIANNOCCARO P, PASTORE C, PÁPAI I, SCHUBERT G. Mechanism of formation of organic carbonates from aliphatic alcohols and carbon dioxide under mild conditions promoted by carbodiimides.DFT calculation and experimental study[J]. J Org Chem,
2005,70(16):6177-6186.
doi: 10.1021/jo050392y
-
[8]
YAMAZAKI Y, KAKUMA K, DU Y, SAITO S. Synthesis of carbonates directly from 1 atm CO2 and alcohols using CH2Cl2[J]. Tetrahedron,
2010,66(51):9675-9680.
doi: 10.1016/j.tet.2010.10.051
-
[9]
KINDERMANN N, JOSE T, KLEIJ A W. Synthesis of carbonates from alcohols and CO2[J]. Top Curr Chem,
2017,375(1)15.
doi: 10.1007/s41061-016-0101-8
-
[10]
SHI F, DENG Y, SIMA T L, PENG J J, GU Y L, QIAO B T. Alternatives to phosgene and carbon monoxide:Synthesis of symmetric urea derivatives with carbon dioxide in ionic liquids[J]. Angew Chem Int Ed,
2003,42(28):3257-3260.
doi: 10.1002/anie.200351098
-
[11]
JIANG T, MA X, ZHOU Y X, LIANG S G, ZHANG J, HAN B X. Solvent-free synthesis of substituted ureas from CO2 and amines with a functional ionic liquid as the catalyst[J]. Green Chem,
2008,10(4):465-469.
doi: 10.1039/b717868a
-
[12]
WU C, CHENG H, LIU R X, WANG Q, HAO Y F, YU Y C, ZHAO F Y. Synthesis of urea derivatives from amines and CO2 in the absence of catalyst and solvent[J]. Green Chem,
2010,12(10):1811-1816.
doi: 10.1039/c0gc00059k
-
[13]
STIEBER S C E, HUGUET N, KAGEYAMA T, JEVTOVIKJ I, ARIYANANDA P, GORDILLO A, SCHUNK S A, ROMINGER F, HOFMANN P, LIMBACH M. Acrylate formation from CO2 and ethylene:Catalysis with palladium and mechanistic insight[J]. Chem Commun,
2015,51(54):10907-10909.
doi: 10.1039/C5CC01932J
-
[14]
KNOPF I, TOFAN D, BEETSTRA D, NEZARI A A, BAHILY K A, CUMMINS C C. A family of cis-macrocyclic diphosphines:Modular, stereoselective synthesis and application in catalytic CO2/ethylene coupling[J]. Chem Sci,
2017,8(2):1463-1468.
doi: 10.1039/C6SC03614G
-
[15]
YU D, ZHANG Y. Copper-and copper-N-heterocyclic carbene-catalyzed C-H activating carboxylation of terminal alkynes with CO2 at ambient conditions[J]. Proc Natl Acad Sci,
2010,107(47):20184-20189.
doi: 10.1073/pnas.1010962107
-
[16]
KIM S H, KIM K H, HONG S H. Carbon dioxide capture and use:organic synthesis using carbon dioxide from exhaust gas[J]. Angew Chem Int Ed,
2014,53(3):771-774.
doi: 10.1002/anie.201308341
-
[17]
JI Dong-feng, WANG Hui, HE Ren. Study on chemical fixation of CO2 and syntheses of alkylene carbonate[J]. J Fuel Chem Technol,
2001,29(6):486-489.
-
[18]
YOSHIDA S, FUKUI K, KIKUCHI S, YAMADA T. Silver-catalyzed enantioselective carbon dioxide incorporation into bispropargylic alcohols[J]. J Am Chem Soc,
2010,132(12):4072-4073.
doi: 10.1021/ja1007118
-
[19]
TAMURA M, HONDA M, NORO K, NAKAGAWA Y, TOMISHIGE K. Heterogeneous CeO2-catalyzed selective synthesis of cyclic carbamates from CO2 and aminoalcohols in acetonitrile solvent[J]. J Catal,
2013,305:191-203.
doi: 10.1016/j.jcat.2013.05.013
-
[20]
HU J, MA J, ZHU Q L, QIAN Q L, HAN H L, MEI Q Q, HAN B X. Zinc (Ⅱ)-catalyzed reactions of carbon dioxide and propargylic alcohols to carbonates at room temperature[J]. Green Chem,
2016,18(2):382-385.
doi: 10.1039/C5GC01870F
-
[21]
YANG Z Z, HE L N, ZHAO Y N, LI B, YU B. CO2 capture and activation by superbase/polyethylene glycol and its subsequent conversion[J]. Energy Environ Sci,
2011,4(10):3971-3975.
doi: 10.1039/c1ee02156g
-
[22]
LIU A H, MA R, SONG C, YANG Z Z, YU A, CAI Y, HE L N, ZHAO Y N, YU B, SONG Q W. Equimolar CO2 capture by N-substituted amino acid salts and subsequent conversion[J]. Angew Chem Int Ed,
2012,51(45):11306-11310.
doi: 10.1002/anie.201205362
-
[23]
BARZAGLI F, LAI S, MANI F. A new class of single-component absorbents for reversible carbon dioxide capture under mild conditions[J]. ChemSusChem,
2015,8(1):184-191.
doi: 10.1002/cssc.201402421
-
[24]
SONG Q W, ZHOU Z H, YIN H, HE L N. Silver(Ⅰ)-catalyzed synthesis of β-oxopropylcarbamates from propargylic alcohols and CO2 surrogate:A gas-free process[J]. ChemSusChem,
2015,8(23):3967-3972.
doi: 10.1002/cssc.201501176
-
[25]
YU B, CHENG B B, LIU W Q, LI W, WANG S S, CAO J, HU C W. Atmospheric pressure of CO2 as protecting reagent and reactant:Efficient synthesis of oxazolidin-2-ones with carbamate salts, aldehydes and alkynes[J]. Adv Synth Catal,
2016,358(1):90-97.
doi: 10.1002/adsc.v358.1
-
[26]
REZAYEE N M, HUFF C A, SANFORD M S. Tandem amine and ruthenium-catalyzed hydrogenation of CO2 to methanol[J]. J Am Chem Soc,
2015,137(3):1028-1031.
doi: 10.1021/ja511329m
-
[27]
SASAKI Y, DIXNEUF P H. Ruthenium-catalyzed reaction of carbon dioxide, amine, and acetylenic alcohol[J]. J Org Chem,
1987,52(19):4389-4391.
doi: 10.1021/jo00228a046
-
[28]
BRUNEAU C, DIXNEUF P H. Catalytic synthesis of O-β-oxoalkylcarbamates[J]. Tetrahedron Lett,
1987,28(18):2005-2008.
doi: 10.1016/S0040-4039(00)96031-3
-
[29]
KIM T J, KWON K H, KWON S C, BAEG J O, SHIM S C. Iron complexes of 1, 1'-bis (diphenylphosphino)ferrocene(BPPF) as efficient catalysts in the synthesis of carbamates.X-ray crystal structure of (BPPF)Fe(CO)3[J]. J Organomet Chem,
1990,389(2):205-217.
doi: 10.1016/0022-328X(90)85412-R
-
[30]
KIM H S, KIM J W, KWON S C, SHIM S C, KIM T J. Catalytic formation of carbamates and cyclic carbonates by copper complex of 2, 5, 19, 22-tetraaza[6, 6] (1, 1')ferrocenophane-1, 5-diene X-ray crystal structure of[Cu(1)]PF6[J]. J Organomet Chem,
1997,545:337-344.
-
[31]
LI Xue-dong, LANG Xian-dong, SONG Qing-wen, GUO Ya-kun, HE Liang-nian. Cu(Ⅰ)-catalyzed three-component reaction of propargylic alcohol, secondary amines and atmospheric CO2[J]. Chin J Org Chem,
2016,36:744-751.
-
[32]
QI C, HUANG L, JIANG H. Efficient synthesis of β-oxoalkyl carbamates from carbon dioxide, internal propargylic alcohols, and secondary amines catalyzed by silver salts and DBU[J]. Synthesis,
2010,41(35):1433-1440.
-
[33]
SONG Q W, YU B, LI X D, MA R, DIAO Z F, LI R G, LI W, HE L N. Efficient chemical fixation of CO2 promoted by a bifunctional Ag2WO4/Ph3P system[J]. Green Chem,
2014,16(3):1633-1638.
doi: 10.1039/c3gc42406e
-
[34]
SONG Q W, CHEN W Q, MA R, YU A, LI Q Y, CHANG Y, HE L N. Bifunctional silver(Ⅰ) complex-catalyzed CO2 conversion at ambient conditions:Synthesis of α-methylene cyclic carbonates and derivatives[J]. ChemSusChem,
2015,8(5):821-827.
doi: 10.1002/cssc.v8.5
-
[35]
SEKINE K, YAMADA T. Silver-catalyzed carboxylation[J]. Chem Soc Rev,
2016,45(16):4524-4532.
doi: 10.1039/C5CS00895F
-
[36]
SONG Q W, LIU P, HAN L H, ZHANG K, HE L N. Upgrading CO2 by incorporation into urethanes through silver-catalyzed one-pot stepwise amidation reaction[J]. Chin J Chem,
2018,36(2):147-152.
doi: 10.1002/cjoc.201700572
-
[37]
CA N D, GABRIELE B, RUFFOLO G, VELTRI L, ZANETTA T, COSTA M. Effective guanidine-catalyzed synthesis of carbonate and carbamate derivatives from propargyl alcohols in supercritical carbon dioxide[J]. Adv Synth Catal,
2011,353(1):133-146.
doi: 10.1002/adsc.201000607
-
[38]
SONG Q W, HE L N. Robust silver(Ⅰ) catalyst for the carboxylative cyclization of propargylic alcohols with carbon dioxide under ambient conditions[J]. Adv Synth Catal,
2016,358(8):1251-1258.
doi: 10.1002/adsc.v358.8

 Login In
Login In






 DownLoad:
DownLoad: