Citation:
Hu Shuxian. Theoretical Studies on Electronic Structure and Chemical Bonding of Actinyl Crown Ether Complexes[J]. Chemistry,
;2020, 83(2): 105-110.

-
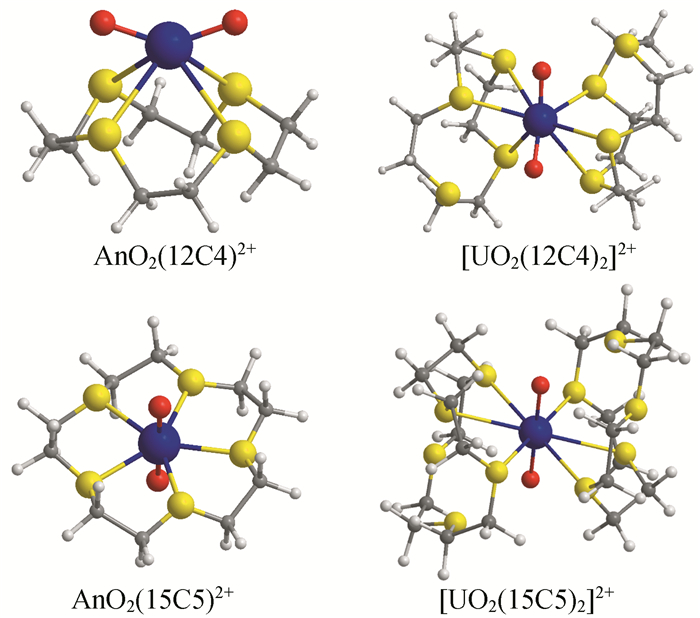
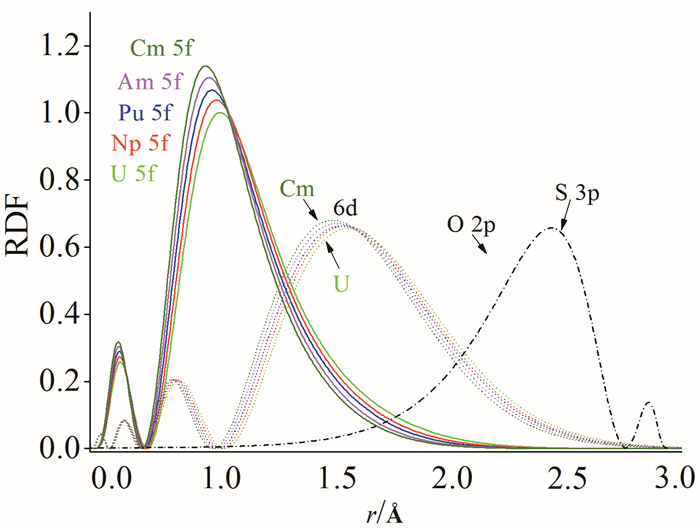
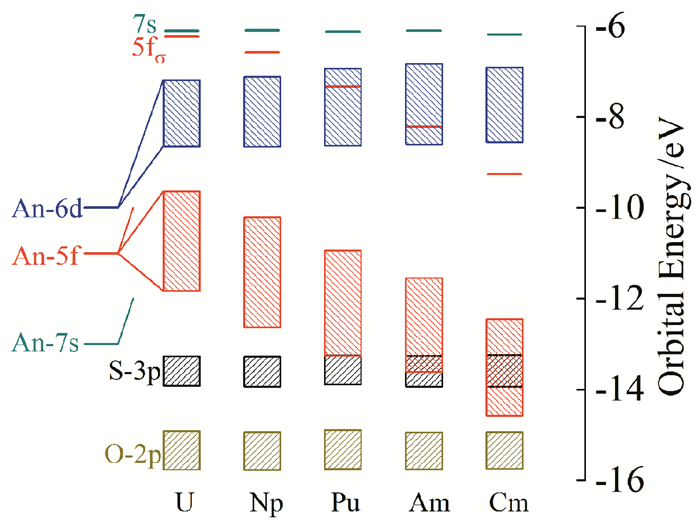
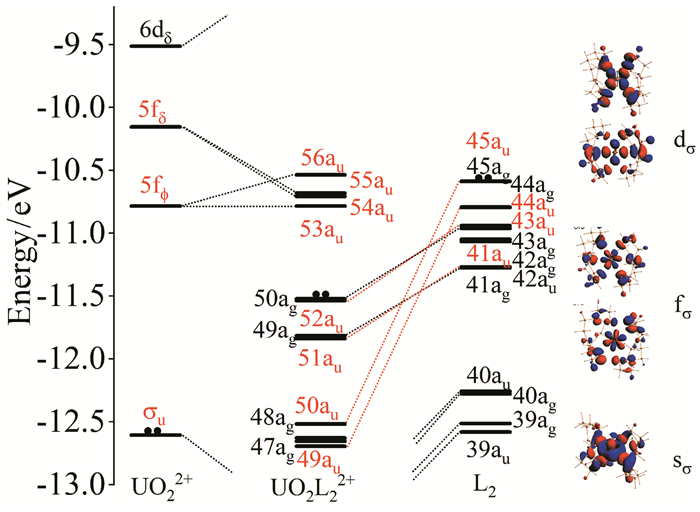
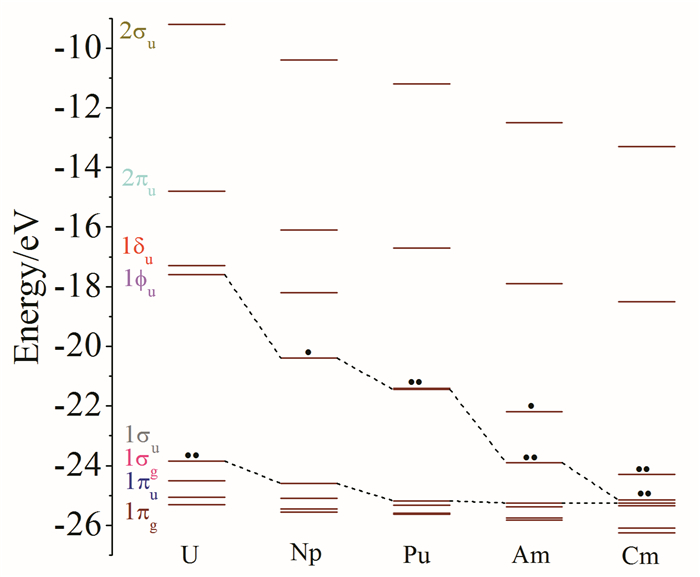
In this mini-review, we have briefly summarized the theoretical research on geometrical structures and coordination chemistry of actinide complexes, the electronic structures of actinyl-crown-ether complexes, and the basic principle for chemical bonding of actinyl complexes. Although increasingly numerous spectroscopy and crystallography data had been available experimentally, there were lack of systematic investigations on theoretically electronic structure and chemical bonding of actinide complexes. Herein reviewed are our recently works from computational chemistry modeling on the coordination structures, stabilization energies and spectra properties of actinyl-crown-ether complexes. Our research has shown that the in-cavity complexes and their bonding features between (thio)-crown ethers and f-elements exhibit conventional conformations, with typical An≡Oactinyl and An-Oligand and An-Sligand distances. The typical ionic An-Oligand and An-Sligand bonds with an extent of covalent interaction between the An and ligand donor atoms primarily attributable to the overlap degree of radial distribution of valence atomic orbitals. From U to Cm, LMCT is gradually significant, resulting in the decrease of the formal oxidation state of Am and Cm, and the weakening of the interaction between metal ions and ligands. This periodicity of chemical bonding and the metal oxidation state provide fundamental guidance for the design of reasonable and efficient ligands in the application of lanthanide-actinide separation.
-

-
- [1]
- [2]
- [3]
-
[4]
Vidaud C, Bourgeois D, Meyer D. Chem. Res. Toxicol., 2012, 25(6): 1161~1175.
- [5]
-
[6]
Gokel G W, Leevy W M, Weber M E. Chem. Rev., 2004, 104: 2723~2750.
-
[7]
Pedersen C J, Frensdor H K. Angew. Chem. Int. Ed., 1972, 11: 16~25.
-
[8]
Cooper T E, Carl D R, Oomens J, et al. J. Phys. Chem. A 2011, 115: 5408~5422.
-
[9]
Servaes K, De Houwer S, Görller-Walrand C, et al. Phys. Chem. Chem. Phys., 2004, 6: 2946~2950.
-
[10]
Shamov G A, Schreckenbach G, Martin R L, et al. Inorg. Chem., 2008, 47: 1465~1475.
- [11]
-
[12]
Hu S X, Gibson J K, Li W L, et al. Chem. Commun., 2016, 52: 12761~12764.
-
[13]
Hu S X, Chen M, Ao B. Phys. Chem. Chem. Phys., 2018, 20: 23856~23863.
-
[14]
Hu S X, Liu H T, Liu J J, et al. ACS Omega, 2018, 3(10): 13902~13912.
-
[15]
Jian J W, Hu S X, Li W L, et al. Inorg. Chem., 2018, 57(7): 4125~4134.
-
[16]
Hu S X, Liu J J, Gibson J K, et al. Inorg. Chem., 2018, 57(5): 2899~2907.
-
[17]
Hu S X, Li L W, Dong L, et al. Dalton Transac., 2017, 46(36): 12354~12363.
-
[18]
Hu S X, Gibson J K, Li W L, et al. Chem. Commun., 2016, 52(86): 12761~12764.
-
[19]
Hu S X, Lu E, Liddle S T. Dalton Transac., 2019, 48(34): 12867~12879.
-
[20]
Qin J, Zhang P, Pu Z, et al. J. Phys. Chem. A, 2019, 123(32): 6958~6969.
-
[21]
Hu S X, Jian J, Li J, et al. Inorg. Chem., 2019, 58(15): 10148~10159.
- [22]
-
[23]
Kovacs A, Konings R J M, Gibson J K, et al. Chem. Rev. 2015, 115(4): 1725~1759.
-
[24]
Marcalo J, Gibson J K. J. Phys. Chem. A, 2009, 113(45): 12599~12606.
-
[25]
Pyykkö P, Li J, Runeberg N. J. Phys. Chem., 1994, 98(18): 4809~4813.
-
[26]
Kovácsa A, Konings R J M. J. Mol. Struct., 2004, 684(1/3): 35~42.
-
[27]
Liu J B. Chen G P. Huang W, et al. Dalton Transac., 2017, 46: 2542~2550.
-
[28]
Su J, Dau P D, Liu H T, et al. J. Chem. Phys., 2015, 142(13): 134308.
-
[29]
Fujii T, Uehara A.; Kitatsuji Y, et al. J. Radioanal. Nucl. Chem., 2015, 303(1): 1015~1020.
-
[30]
Lan J H, Wang C Z, Wu Q Y, et al. J. Phys. Chem. A, 2015, 119(34): 9178~9188.
- [31]
-
[32]
Wang Y L, Liu Z Y, Li Y X, et al. J. Am. Chem. Soc., 2015, 137(19): 6144~6147.
-
[33]
Neuefeind J, Soderholm L, Skanthakumar S. J. Phys. Chem. A, 2004, 108: 2733~2739.
-
[34]
Kovács A, Pogany P, Konings R. Inorg. Chem., 2012, 51(8): 4841~4849.
-
[35]
Sokolova M N, Fedosseev A M, Andreev G B, et al. Inorg. Chem., 2009, 48(19): 9185~9190.
-
[36]
Burns C J, Bursten B E. Comment. Inorg. Chem., 1989, 9(2): 61~93.
-
[37]
Bursten B E, Palmer E J, Sonnenberg J L. Special Publication-Royal Society of Chemistry, 2006, 305(1): 157~162.
-

-
-
[1]
Yanan Jiang , Yuchen Ma . Brief Discussion on the Electronic Exchange Interaction in Quantum Chemistry Computations. University Chemistry, 2025, 40(3): 10-15. doi: 10.12461/PKU.DXHX202402058
-
[2]
Zhenming Xu , Mingbo Zheng , Zhenhui Liu , Duo Chen , Qingsheng Liu . Experimental Design of Project-Driven Teaching in Computational Materials Science: First-Principles Calculations of the LiFePO4 Cathode Material for Lithium-Ion Batteries. University Chemistry, 2024, 39(4): 140-148. doi: 10.3866/PKU.DXHX202307022
-
[3]
Linfeng Xiao , Wanlu Ren , Shishi Shen , Mengshan Chen , Runhua Liao , Yingtang Zhou , Xibao Li . Enhancing Photocatalytic Hydrogen Evolution through Electronic Structure and Wettability Adjustment of ZnIn2S4/Bi2O3 S-Scheme Heterojunction. Acta Physico-Chimica Sinica, 2024, 40(8): 2308036-0. doi: 10.3866/PKU.WHXB202308036
-
[4]
Xuyang Wang , Jiapei Zhang , Lirui Zhao , Xiaowen Xu , Guizheng Zou , Bin Zhang . Theoretical Study on the Structure and Stability of Copper-Ammonia Coordination Ions. University Chemistry, 2024, 39(3): 384-389. doi: 10.3866/PKU.DXHX202309065
-
[5]
Baitong Wei , Jinxin Guo , Xigong Liu , Rongxiu Zhu , Lei Liu . Theoretical Study on the Structure, Stability of Hydrocarbon Free Radicals and Selectivity of Alkane Chlorination Reaction. University Chemistry, 2025, 40(3): 402-407. doi: 10.12461/PKU.DXHX202406003
-
[6]
Jia Zhou . Design and Practice of a Comprehensive Computational Chemistry Experiment Based on High-Throughput Computation and Machine Learning. University Chemistry, 2025, 40(9): 69-75. doi: 10.12461/PKU.DXHX202411067
-
[7]
Hongting Yan , Aili Feng , Rongxiu Zhu , Lei Liu , Dongju Zhang . Reexamination of the Iodine-Catalyzed Chlorination Reaction of Chlorobenzene Using Computational Chemistry Methods. University Chemistry, 2025, 40(3): 16-22. doi: 10.12461/PKU.DXHX202403010
-
[8]
Tongqi Ye , Yanqing Wang , Qi Wang , Huaiping Cong , Xianghua Kong , Yuewen Ye . Reform of Classical Thermodynamics Curriculum from the Perspective of Computational Chemistry. University Chemistry, 2025, 40(7): 387-392. doi: 10.12461/PKU.DXHX202409128
-
[9]
Renqing Lü , Shutao Wang , Fang Wang , Guoping Shen . Computational Chemistry Aided Organic Chemistry Teaching: A Case of Comparison of Basicity and Stability of Diazine Isomers. University Chemistry, 2025, 40(3): 76-82. doi: 10.12461/PKU.DXHX202404119
-
[10]
Yi Li , Zhaoxiang Cao , Peng Liu , Xia Wu , Dongju Zhang . Revealing the Coloration and Color Change Mechanisms of the Eriochrome Black T Indicator through Computational Chemistry and UV-Visible Absorption Spectroscopy. University Chemistry, 2025, 40(3): 132-139. doi: 10.12461/PKU.DXHX202405154
-
[11]
Yiying Yang , Dongju Zhang . Elucidating the Concepts of Thermodynamic Control and Kinetic Control in Chemical Reactions through Theoretical Chemistry Calculations: A Computational Chemistry Experiment on the Diels-Alder Reaction. University Chemistry, 2024, 39(3): 327-335. doi: 10.3866/PKU.DXHX202309074
-
[12]
Xu Li , Qinglan Li , Qingji Wang . Research and Practice of Computational Chemistry in Inorganic Chemistry Education. University Chemistry, 2025, 40(7): 345-351. doi: 10.12461/PKU.DXHX202408114
-
[13]
Qian Huang , Zhaowei Li , Jianing Zhao , Ao Yu . Quantum Chemical Calculations Reveal the Details Below the Experimental Phenomenon. University Chemistry, 2024, 39(3): 395-400. doi: 10.3866/PKU.DXHX202309018
-
[14]
Congying Lu , Fei Zhong , Zhenyu Yuan , Shuaibing Li , Jiayao Li , Jiewen Liu , Xianyang Hu , Liqun Sun , Rui Li , Meijuan Hu . Experimental Improvement of Surfactant Interface Chemistry: An Integrated Design for the Fusion of Experiment and Simulation. University Chemistry, 2024, 39(3): 283-293. doi: 10.3866/PKU.DXHX202308097
-
[15]
Hao XU , Ruopeng LI , Peixia YANG , Anmin LIU , Jie BAI . Regulation mechanism of halogen axial coordination atoms on the oxygen reduction activity of Fe-N4 site: A density functional theory study. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 695-701. doi: 10.11862/CJIC.20240302
-
[16]
Junqing WEN , Ruoqi WANG , Jianmin ZHANG . Regulation of photocatalytic hydrogen production performance in GaN/ZnO heterojunction through doping with Li and Au. Chinese Journal of Inorganic Chemistry, 2025, 41(5): 923-938. doi: 10.11862/CJIC.20240243
-
[17]
Huiwei Ding , Bo Peng , Zhihao Wang , Qiaofeng Han . Advances in Metal or Nonmetal Modification of Bismuth-Based Photocatalysts. Acta Physico-Chimica Sinica, 2024, 40(4): 2305048-0. doi: 10.3866/PKU.WHXB202305048
-
[18]
Shule Liu . Application of SPC/E Water Model in Molecular Dynamics Teaching Experiments. University Chemistry, 2024, 39(4): 338-342. doi: 10.3866/PKU.DXHX202310029
-
[19]
Qiwen Chen , Baolei Wang . Research Progress on One-Electron σ-Bond of Organic Compounds. University Chemistry, 2025, 40(11): 191-198. doi: 10.12461/PKU.DXHX202412136
-
[20]
Zhaoyang WANG , Chun YANG , Yaoyao Song , Na HAN , Xiaomeng LIU , Qinglun WANG . Lanthanide(Ⅲ) complexes derived from 4′-(2-pyridyl)-2, 2′∶6′, 2″-terpyridine: Crystal structures, fluorescent and magnetic properties. Chinese Journal of Inorganic Chemistry, 2024, 40(8): 1442-1451. doi: 10.11862/CJIC.20240114
-
[1]
Metrics
- PDF Downloads(27)
- Abstract views(1466)
- HTML views(374)

 Login In
Login In




 DownLoad:
DownLoad: