Citation:
ZHANG Xiu-xia, LÜ Xiao-xue, XIAO Mei-hua, LIN Ri-yi, ZHOU Zhi-jun. Molecular reaction dynamics simulation of pyrolysis mechanism of typical bituminous coal via ReaxFF[J]. Journal of Fuel Chemistry and Technology,
;2020, 48(9): 1035-1046.

-
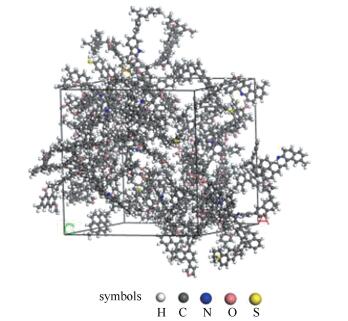
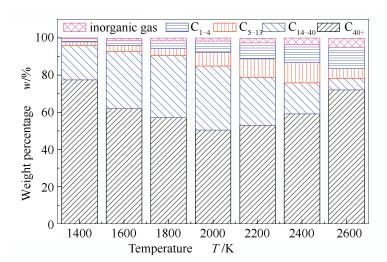
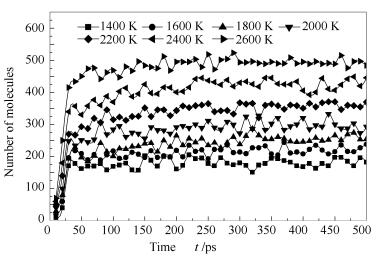
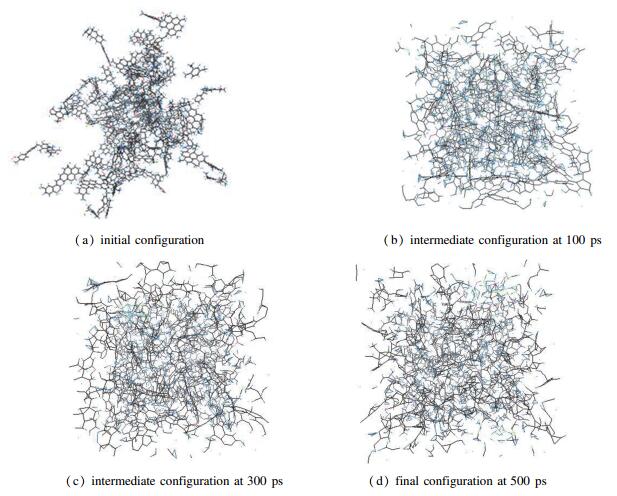
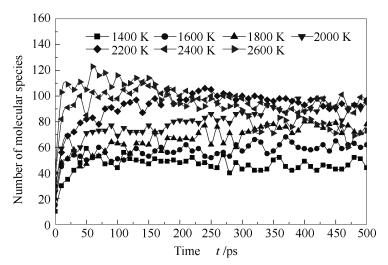
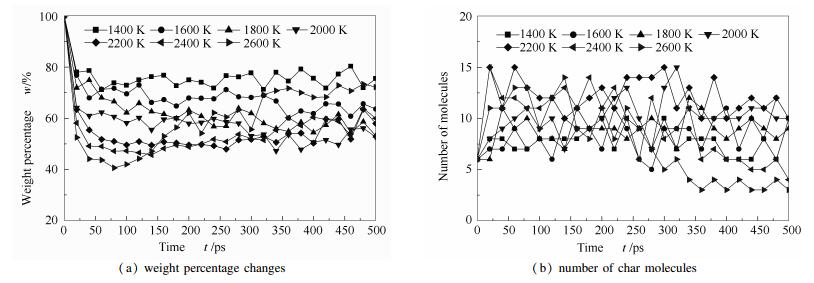
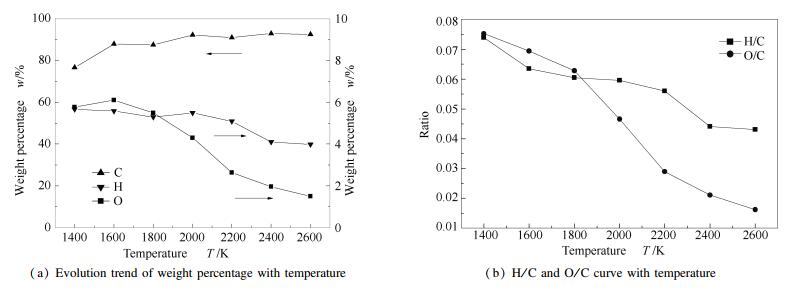
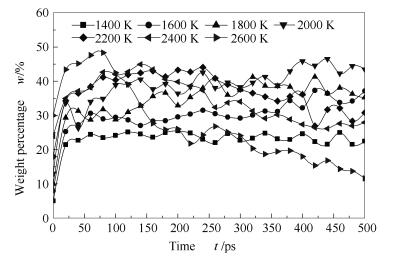
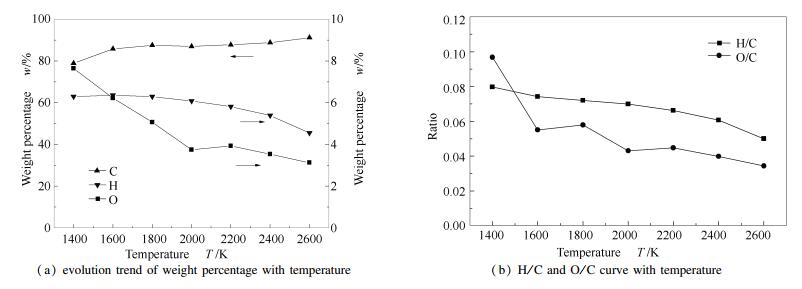
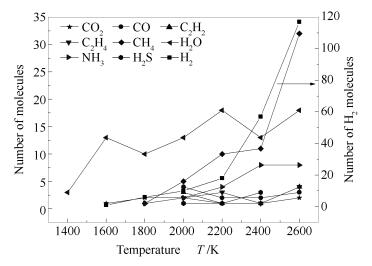
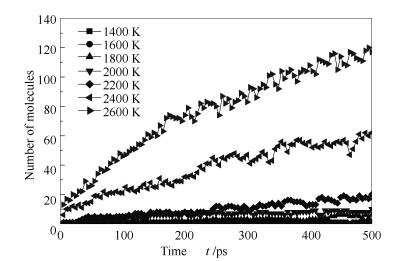
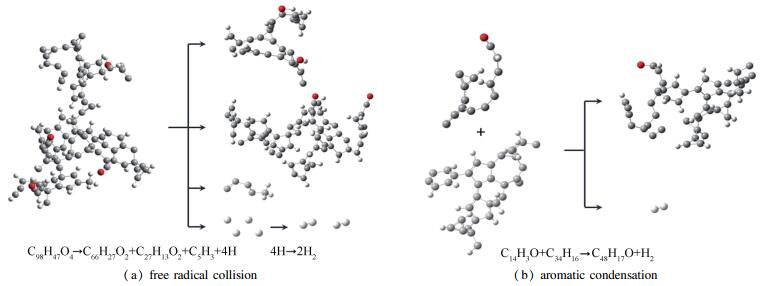
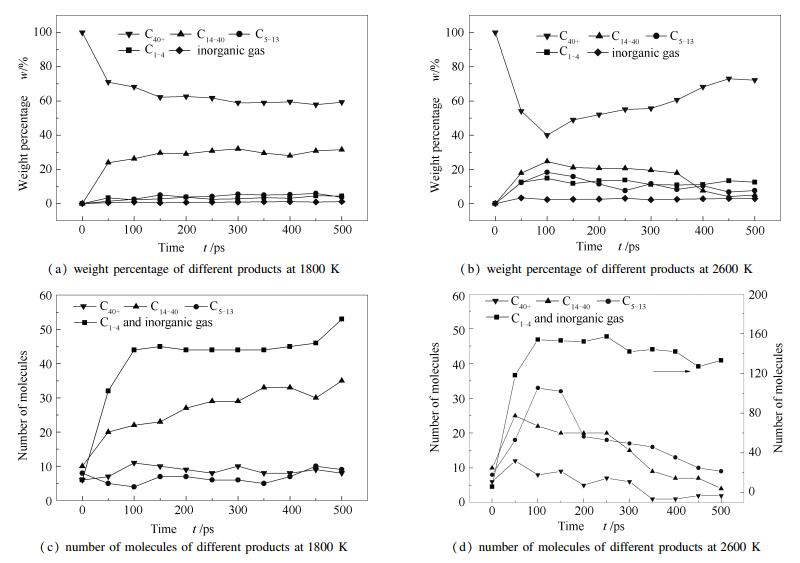
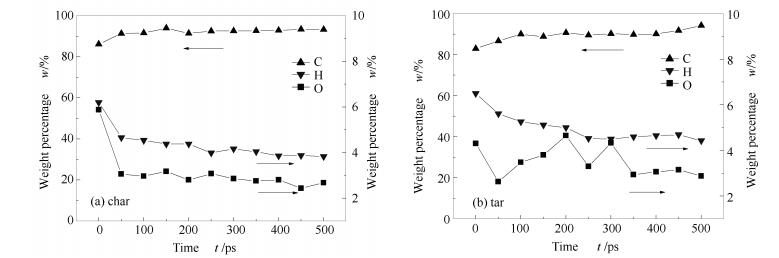
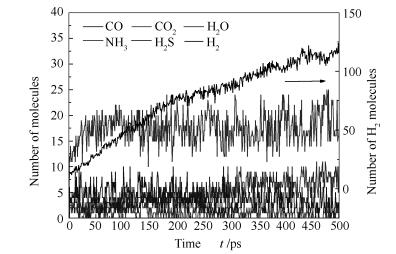
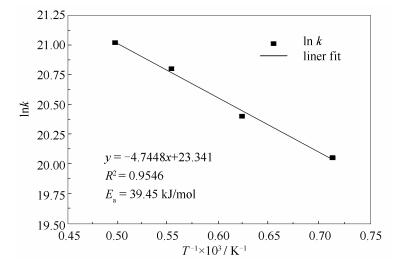
A reasonable and effective macromolecular model of bituminous coal was established. The molecular dynamics method based on reactive force field (ReaxFF) was used to simulate the pyrolysis process of typical bituminous coal in the range of 1400-2600 K. The distribution of products and evolution of intermediate radicals were analyzed. Calculation results showed that with increase of pyrolysis temperature, yield of char firstly increased and then decreased, while the trend in tar production was opposite. Yield of pyrolysis gas increased monotonously with increasing temperature. The pyrolysis of coal at low temperature mainly experienced primary reaction with formation of tar free radical fragments and small molecular gases. At high temperature, the secondary reaction of tar fragments was remarkable, and char with more content but less quantity and small molecular gas with more content and quantity were produced. The temperature turning point from the primary reaction to the second one was 2000 K. Under the high temperature pyrolysis conditions, C and H in coal gradually migrated into char and tar, while oxygen-containing functional groups were more active, resulting in migration of O to pyrolysis gases. In the secondary reaction stage, comparing chemical properties of the three elements C, H and O, O was the most active, H was the second, and C was the most stable. H2O was firstly released during pyrolysis. NH3 mainly came from secondary reactions during which H2S was consumed and converted into other products. Yield of H2 was the highest, and increased with increasing pyrolysis temperature. A large amount of H2 was generated in secondary reactions, which was mainly from collision of hydrogen radicals generated from pyrolysis and condensation of aromatic structures. Based on ReaxFF simulation results, the weightless activation energy of coal pyrolysis was 39.45 kJ/mol.
-
Keywords:
- bituminous coal,
- pyrolysis,
- char,
- tar,
- activation energy,
- ReaxFF
-

-
-
[1]
YUE Guang-xi, ZHOU Da-li, TIAN Wen-long, MA Lin-wei, LIU Qing, ZHANG Jing-hao, WANG Zhi-xuan, LONG Hui, LIAO Hai-yan. Preliminary discussion on the technology roadmap of clean coal combustion in China[J]. Eng Sci, 2018,20(3):74-79.
-
[2]
CHEN Zhao-hui, DUN Qi-meng, SHI Yong, GAO Shi-qiu. Effects of pyrolysis temperature and atmosphere on rapid coal pyrolysis in transport bed reactor[J]. CIESC J, 2017,68(4):1566-1573.
-
[3]
CHANG Qing-hua, LI Hong-jun, CUI Tong-min, FAN Wen-ke, YU Guang-suo, WANG Fu-chen. Effect of moisture content on gas release and pore structure development of wetted Shenfu coal during rapid pyrolysis[J]. J Fuel Chem Technol, 2017,45(4):427-435.
-
[4]
YANG Zhi-rong, MENG Qing-yan, HUANG Jie-jie, WANG Zhi-qing, LI Chun-yu, FANG Yi-tian. Interaction between Shenmu coal and different caking coals during co-pyrolysis[J]. J Fuel Chem Technol, 2018,46(6):641-648.
-
[5]
ZHAO Ning, LIU Dong, ZHAO Meng-meng, ZHANG Zhi-chen, XIANG Zai-jin. Rotary pyrolysis characteristic of low rank coal from northern Shaanxi[J]. J China Univ Pet, 2019,43(3):167-175.
-
[6]
HE W J, LIU Z Y, LIU Q Y, CI D H, LIEVENS C, GUO X F. Behaviors of radical fragments in tar generated from pyrolysis of 4 coals[J]. Fuel, 2014,134:375-380. doi: 10.1016/j.fuel.2014.05.064
-
[7]
GODDARD W A, MERINOV B, van DUIN A C T, JACOB T, BLANCO M, MOLINERO V, JANG S S, JANG Y H. Multi-paradigm multi-scale simulations for fuel cell catalysts and membranes[J]. Mol Simul, 2006,32(3/4):251-268.
-
[8]
ZHANG X X, XIE M, WU H X, LÜ X X, ZHOU Z J. DFT study of the effect of Ca on NO heterogeneous reduction by char[J]. Fuel, 2020,265116995. doi: 10.1016/j.fuel.2019.116995
-
[9]
ZHANG X X, WU H X, XIE M, LÜ X X, ZHOU Z J, LIN R Y. A thorough theoretical exploration of the effect mechanism of Fe on HCN heterogeneous formation from nitrogen-containing char[J]. Fuel, 2020,280118662. doi: 10.1016/j.fuel.2020.118662
-
[10]
XÜ Zi-yang, YUE Shuang, WANG Chun-bo, LIU Rui-qi. Reaction mechanism of NO reduction with CO catalyzed by char[J]. J Fuel Chem Technol, 2020,48(3):266-274.
-
[11]
LI Zhi-peng, NIU Sheng-li, ZHAO Gai-ju, HAN Kui-hua, LI Ying-jie, LU Chun-mei, CHENG Shen. Molecular simulation study of strontium doping on the adsorption of methanol on CaO (100) surface[J]. J Fuel Chem Technol, 2020,48(2):172-178.
-
[12]
DENG Jun, LI Ya-qing, ZHANG Yu-tao, YANG Chao-ping, ZHANG Jing, SHI Xue-qiang. Effects of hydroxyl on oxidation characteristics of side chain active groups in coal[J]. J China Coal Soc, 2020,45(1):232-240.
-
[13]
HOU Ying-fei, JIANG Chi, LI Li-jun, LIU En-jie, LIN Peng-fei, NIU Qing-shan. Diffusion behavior of gasoline components in crosslinked ethyl cellulose by molecular dynamics simulation[J]. J China Univ Pet, 2018,42(1):171-176.
-
[14]
AGRAWALLA S, VAN DUIN A C T. Development and application of a reaxff reactive force field for hydrogen combustion[J]. J Phys Chem A, 2011,115(6):960-972. doi: 10.1021/jp108325e
-
[15]
ZHANG L, ZYBIN S V, VAN DUIN A C T, DASGUPTA S, GODDARD W A. Carbon cluster formation during thermal decomposition of octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine and1, 3, 5-triamino-2, 4, 6-trinitrobenzene high explosives from Reax FF reactive molecular dynamics simulations[J]. J Phys Chem A, 2009,113(40):10619-10640. doi: 10.1021/jp901353a
-
[16]
MONTI S, COROZZI A, FRISTRUP P, JOSHI K L, SHIN Y K, OELSCHLAEGER P, VAN DUIN A C T, BARONE V. Exploring the conformational and reactive dynamics of biomolecules in solution using an extended version of the glycine reactive force field[J]. Phys Chem Chem Phys, 2013,15(36):15062-15077. doi: 10.1039/c3cp51931g
-
[17]
SENFTLE T P, HONG S, ISLAM M M, KYLASA S B, ZHENG Y X, SHIN Y K, JUNKERMEIER C, ENGEL-HERBERT R, JANIK M J, AKTULGA H M, VERSTRAELEN T, GRAMA A, VAN DUIN A C T. The ReaxFF reactive force-field:Development, applications and future directions[J]. NPJ Comput Mater, 2016,2(1):9396-9409.
-
[18]
CASTRO-MARCANO F, RUSSO M F, VAN DUIN A C T, MATHEWS J P. Pyrolysis of a large-scale molecular model for Illinois no. 6 coal using the Reax FF reactive force field[J]. J Anal Appl Pyrolysis, 2014(109):79-89.
-
[19]
CASTRO-MARCANO F, KAMAT A M, RUSSO M F, van DUIN A C T, MATHEWS J P. Combustion of an Illinois No. 6 coal char simulated using an atomistic char representation and the Reax FF reactive force field[J]. Combust Flame, 2011,159(3):1272-1285.
-
[20]
ZHENG M, LI X X, LIU J, WANG Z, GONG X M, GUO L, SONG W L. Pyrolysis of Liulin coal simulated by GPU-Based ReaxFF MD with cheminformatics analysis[J]. Energy Fuels, 2014,28(1):522-534. doi: 10.1021/ef402140n
-
[21]
ZHENG M, YANG P, WANG Z, LI X X, LI G. Capturing the dynamic profiles of products in Hailaer brown coal pyrolysis with reactive molecular simulations and experiments[J]. Fuel, 2020268.
-
[22]
GAO M J, LI X X, GUO L. Pyrolysis simulations of Fugu coal by large-scale ReaxFF molecular dynamics[J]. Fuel Process Technol, 2018,178:197-205. doi: 10.1016/j.fuproc.2018.05.011
-
[23]
HONG D K, GUO X. Molecular dynamics simulations of Zhundong coal pyrolysis using reactive force field[J]. Fuel, 2017,210(1):58-66.
-
[24]
HONG D K, LIU L, ZhANG S, GUO X. Effect of cooling rate on the reaction of volatiles from low-rank coal pyrolysis:Molecular dynamics simulations using ReaxFF[J]. Fuel Process Technol, 2018,178:133-138. doi: 10.1016/j.fuproc.2018.05.033
-
[25]
ZHOU Xing-yu, ZENG Fan-gui, XIANG Jian-hua, DENG Xiao-peng, XIANG Xing-hua. Macromolecular model construction and molecular simulation of organic matter in Majiliang vitrain[J]. CIESC J, 2020,71(4):1802-1811.
-
[26]
GAO Ming-jie. Pyrolysis simulation of Fugu subbituminous coal by reaxFF molecular dynamics[D]. Beijing: Institute of Process Engineering, Chinese Academy of Sciences, 2019.
-
[27]
ZHENG M, LI X X, GUO L. Investigation of N behavior during coal pyrolysis and oxidation using ReaxFF molecular dynamics[J]. Fuel, 2018,233:867-876. doi: 10.1016/j.fuel.2018.06.133
-
[28]
GAO Ning, WANG Yi-chao, LIU Yu-hong. Molecular dynamics simulations of thermal pyrolysis of novel dipropargyl ether of bisphenol A based boron-containing polymer[J]. CIESC J, 2015,66(4):1557-1564.
-
[29]
YUAN Ming, LIN Hua-lin, LI Ke-jian. Coal macromolecular structure models and relevant research methods[J]. Clean Coal Technol, 2013,19(2):42-46.
-
[30]
WISER W H, ANDERSON L L, QADER S A, HILL G R. Kinetic relationship of coal hydrogenation, pyrolysis and dissolution[J]. J Chem Technol Biot, 1971,21(3):82-86.
-
[31]
ZHENG Mo. Coal pyrolysis simulation by GPU-based reactive force field molecular dynamics (ReaxFF MD)[D]. Beijing: Institute of Process Engineering, Chinese Academy of Sciences, 2015.
-
[32]
MIURA K, SHIMADA M, MAE K, SOCK H Y. Extraction of coal below 350℃ in flowing non-polar solvent[J]. Fuel, 2001,80(11):1573-1582. doi: 10.1016/S0016-2361(01)00036-9
-
[33]
GONG X M, WANG Z, LI S G, SONG W L, LIN W G. Coal pyrolysis in a laboratory-scale two-stage reactor:Catalytic upgrading of pyrolytic vapors[J]. Chem Eng Technol, 2014,37(12).
-
[34]
GONG X M, WANG Z, DENG S W, LI S G, SONG W L, LIN W G. Impact of the temperature, pressure, and particle size on tar composition from pyrolysis of three ranks of Chinese coals[J]. Energy Fuels, 2014,28(8):4942-4948. doi: 10.1021/ef500986h
-
[35]
ZHENG M, LI X X, LIU J, GUO L. Initial chemical reaction simulation of coal pyrolysis via ReaxFF molecular dynamics[J]. Energy Fuels, 2013,27(6):2942-2951. doi: 10.1021/ef400143z
-
[36]
DONG T, MURATA S, MIURA M, NOMURA M, NAKAMURA K. Computer-aided molecular design study of coal model molecules. 3. Density simulation for model structures of bituminous Akabira coal[J]. Energy Fuels, 2002,7(6):1123-1127.
-
[37]
NAKAMURA K, MURATA S, NOMURA M. CAMD study of coal model molecules. 1. Estimation of physical density of coal model molecules[J]. Energy Fuels, 2002,7(3):347-350.
-
[38]
VAN DUIN A C T, DASGUPTA S, LORANT F, GODDARD W A. ReaxFF:A reactive force field for hydrocarbons[J]. J Phys Chem A, 2001,105(41):9396-9409. doi: 10.1021/jp004368u
-
[39]
HONG Di-kun. Study on the pyrolysis and oxy-fuel combustion of Zhundong coal using reactive molecular dynamics simulations[D]. Wuhan: Huazhong University of Science and Technology, 2018.
-
[40]
HONG Di-kun, CAO Zheng, YANG Chang-min, LIU Liang, GUO Xin. Catalytic effect of calcium on reaction of phenol using reactive molecular dynamics simulation[J]. CIESC J, 2019,70(5):1788-1794.
-
[41]
ZHENG M, LI X X, WANG M J, GUO L. Dynamic profiles of tar products during Naomaohu coal pyrolysis revealed by large-scale reactive molecular dynamic simulation[J]. Fuel, 2019,253:910-920. doi: 10.1016/j.fuel.2019.05.085
-
[42]
HONG Di-kun, LIU Liang, CAO Zheng, YANG Chang-min, GUO Xin. Molecular dynamics simulation of Wucaiwan coal pyrolysis via ReaxFF[J]. J China Coal Soc, 2019,44(S1):271-277.
-
[43]
LEI Zhao, YANG Ding, ZHANG Yun-He, CUI Ping. Constructions of coal and char molecular models based on the molecular simulation technology[J]. J Fuel Chem Technol, 2017,45(7):769-779.
-
[44]
HUMPHREY W, DALKE A, SCHULTEN K. VMD:visual molecular dynamics[J]. J Mol Graphics Modell, 1996,14:33-38. doi: 10.1016/0263-7855(96)00018-5
-
[45]
SOLOMON P R, FLETCHER T H, PUGMIRE R J. Progress in coal pyrolysis[J]. Fuel, 1993,72(5):587-597. doi: 10.1016/0016-2361(93)90570-R
-
[46]
WANG Lian-cong, LIANG Yun-tao. Spectral analysis on laws of generation and variability of CO during oxygen-free programmed temperature of coal[J]. J China Coal Soc, 2017,42(7):1790-1794.
-
[47]
LIU J X, JIANG X M, SHEN J, ZHANG H. Pyrolysis of superfine pulverized coal. Part 1. Mechanisms of methane formation[J]. Energ Convers Manage, 2014,87:1027-1038. doi: 10.1016/j.enconman.2014.07.053
-
[48]
DOMAZETIS G, JAMES B D. Molecular models of brown coal containing inorganic species[J]. Org Geochem, 2007,37(2):244-259.
-
[49]
MAO Ning, WANG Qiang, YANG Yan, XU Dun-xin, FENG Wei, ZHANG Jin-peng, BAI Hong-cun, GUO Qing-jie. Pyrolysis characteristics and kinetics analysis of Qinghua coal, Ningxia based on chemical bonding characteristics of macerals[J]. CIESC J, 2020,71(2):811-820+903.
-
[50]
WU Jie, DI Zuo-xing, LUO Ming-sheng, WANG Ya-tao, DING Xiao-xiao. Study of the effects of temperature and pressure on the coal pyrolysis in the atmosphere of N2[J]. Chem Ind Eng Prog, 2019, 38(S1): 116-121.
-
[51]
LIU Z T, ZHAN J H, LAI D G, ZUO M H, LIU X X. Effects of different temperature chars on distribution of pyrolysates for Naomaohu coal[J]. J Therm Anal Calorim, 2020.
-
[52]
DENG Yi-ying. The pyrolysis experiment study of pingshuo coal[J]. Clean Coal Technology, 2008,14(2):56-58, 66.
-
[53]
SOLOMON P R, SERIO M A, SUUBERG E M. Coal pyrolysis:Experiments, kinetic rates and mechanisms[J]. Prog Energy Combust Sci, 1992,18(2):133-220. doi: 10.1016/0360-1285(92)90021-R
-
[1]
-

-
-
[1]
Zihan Cheng , Kai Jiang , Jun Jiang , Henggang Wang , Hengwei Lin . Achieving thermal-stimulus-responsive dynamic afterglow from carbon dots by singlet-triplet energy gap engineering through covalent fixation. Acta Physico-Chimica Sinica, 2026, 42(2): 100169-0. doi: 10.1016/j.actphy.2025.100169
-
[2]
Rohit Kumar , Anita Sudhaik , Aftab Asalam Pawaz Khan , Van Huy Neguyen , Archana Singh , Pardeep Singh , Sourbh Thakur , Pankaj Raizada . Designing tandem S-scheme photo-catalytic systems: Mechanistic insights, characterization techniques, and applications. Acta Physico-Chimica Sinica, 2025, 41(11): 100150-0. doi: 10.1016/j.actphy.2025.100150
-
[3]
Kexin Yan , Zhaoqi Ye , Lingtao Kong , He Li , Xue Yang , Yahong Zhang , Hongbin Zhang , Yi Tang . Seed-Induced Synthesis of Disc-Cluster Zeolite L Mesocrystals with Ultrashort c-Axis: Morphology Control, Decoupled Mechanism, and Enhanced Adsorption. Acta Physico-Chimica Sinica, 2024, 40(9): 2308019-0. doi: 10.3866/PKU.WHXB202308019
-
[4]
Zhuo WANG , Junshan ZHANG , Shaoyan YANG , Lingyan ZHOU , Yedi LI , Yuanpei LAN . Preparation and photocatalytic performance of CeO2-reduced graphene oxide by thermal decomposition. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1708-1718. doi: 10.11862/CJIC.20240067
-
[5]
Yang Lv , Yingping Jia , Yanhua Li , Hexiang Zhong , Xinping Wang . Integrating the Ideological Elements with the “Chemical Reaction Heat” Teaching. University Chemistry, 2024, 39(11): 44-51. doi: 10.12461/PKU.DXHX202402059
-
[6]
Yang ZHOU , Lili YAN , Wenjuan ZHANG , Pinhua RAO . Thermal regeneration of biogas residue biochar and the ammonia nitrogen adsorption properties. Chinese Journal of Inorganic Chemistry, 2025, 41(8): 1574-1588. doi: 10.11862/CJIC.20250032
-
[7]
Zehua Zhao , Xiaoyan An , Jinrong Xu , Ling Yang , Hao Zhao , Zhongyun Wu . Independent Development and Application of Calorimetric Experiment Data Acquisition and Processing Software. University Chemistry, 2025, 40(11): 402-408. doi: 10.12461/PKU.DXHX202505045
-
[8]
Limei CHEN , Mengfei ZHAO , Lin CHEN , Ding LI , Wei LI , Weiye HAN , Hongbin WANG . Preparation and performance of paraffin/alkali modified diatomite/expanded graphite composite phase change thermal storage material. Chinese Journal of Inorganic Chemistry, 2024, 40(3): 533-543. doi: 10.11862/CJIC.20230312
-
[9]
Yuting Bai , Cenqi Yan , Zhen Li , Jiaqiang Qin , Pei Cheng . Preparation of High-Strength Polyimide Porous Films with Thermally Closed Pore Property by In Situ Pore Formation Method. Acta Physico-Chimica Sinica, 2024, 40(9): 2306010-0. doi: 10.3866/PKU.WHXB202306010
-
[10]
Yongqing Xu , Yuyao Yang , Mengna Wu , Xiaoxiao Yang , Xuan Bie , Shiyu Zhang , Qinghai Li , Yanguo Zhang , Chenwei Zhang , Robert E. Przekop , Bogna Sztorch , Dariusz Brzakalski , Hui Zhou . Review on Using Molybdenum Carbides for the Thermal Catalysis of CO2 Hydrogenation to Produce High-Value-Added Chemicals and Fuels. Acta Physico-Chimica Sinica, 2024, 40(4): 2304003-0. doi: 10.3866/PKU.WHXB202304003
-
[11]
Chengpeng Liu , Yinxia Fu . Design and Practice of Ideological and Political Education for the Public Elective Course “Life Chemistry Experiment” in Universities. University Chemistry, 2024, 39(10): 242-248. doi: 10.12461/PKU.DXHX202404064
-
[12]
Fa Wang , Yu Chen , Hui Chao . Ruthenium(II) Complexes as Photoactivated Chemo-Prodrugs for Hypoxic Tumor Therapy. University Chemistry, 2025, 40(7): 200-212. doi: 10.12461/PKU.DXHX202410024
-
[13]
Xue-Peng Zhang , Yuchi Long , Yushu Pan , Jiding Wang , Baoyu Bai , Rui Ding . 定量构效关系方法学习探索:以钴卟啉活化氧气为例. University Chemistry, 2025, 40(8): 345-359. doi: 10.12461/PKU.DXHX202410107
-
[14]
Dan LUO , Xingcheng LIU , Dong LI , Tong CHANG . Metal-support interaction effects on CO activation over Con/SiO2 catalysts. Chinese Journal of Inorganic Chemistry, 2025, 41(11): 2337-2344. doi: 10.11862/CJIC.20250003
-
[15]
Yahui HAN , Jinjin ZHAO , Ning REN , Jianjun ZHANG . Synthesis, crystal structure, thermal decomposition mechanism, and fluorescence properties of benzoic acid and 4-hydroxy-2, 2′: 6′, 2″-terpyridine lanthanide complexes. Chinese Journal of Inorganic Chemistry, 2025, 41(5): 969-982. doi: 10.11862/CJIC.20240395
-
[16]
.
CCS Chemistry | 超分子活化底物为自由基促进高效选择性光催化氧化
. CCS Chemistry, 2025, 7(10.31635/ccschem.025.202405229): -. -
[17]
Yunxin Xu , Wenbo Zhang , Jing Yan , Wangchang Geng , Yi Yan . A Fascinating Saga of “Energetic Materials”. University Chemistry, 2024, 39(9): 266-272. doi: 10.3866/PKU.DXHX202307008
-
[18]
Jianjun LI , Mingjie REN , Lili ZHANG , Lingling ZENG , Huiling WANG , Xiangwu MENG . UV-assisted degradation of tetracycline hydrochloride by MnFe2O4@activated carbon activated persulfate. Chinese Journal of Inorganic Chemistry, 2024, 40(10): 1869-1880. doi: 10.11862/CJIC.20240187
-
[19]
Xinxin YU , Yongxing LIU , Xiaohong YI , Miao CHANG , Fei WANG , Peng WANG , Chongchen WANG . Photocatalytic peroxydisulfate activation for degrading organic pollutants over the zero-valent iron recovered from subway tunnels. Chinese Journal of Inorganic Chemistry, 2025, 41(5): 864-876. doi: 10.11862/CJIC.20240438
-
[20]
Yongxin LIU , Xingchen LI , Hongjia LIU , Danni LI , Tao ZHANG , Xi CHEN . Enhancement effect of Fe3O4 conversion to MIL-100(Fe) on activation of persulfate for degradation of antibiotic. Chinese Journal of Inorganic Chemistry, 2025, 41(12): 2503-2513. doi: 10.11862/CJIC.20250169
-
[1]
Metrics
- PDF Downloads(17)
- Abstract views(2284)
- HTML views(568)

 Login In
Login In




 DownLoad:
DownLoad: